PHOTONICS APPLIED: THERANOSTICS: Medical diagnostics and therapy combine: Theranostics arrives
The inaugural issue of the open-access journal Theranostics (www.thno.org) was launched in early 2011 and described by its editor-in-chief Xiaoyuan (Shawn) Chen, Sr., investigator and chief of the Laboratory of Molecular Imaging and Nanomedicine at the National Institutes of Health (Bethesda, MD), as “a forum for the exchange of clinical and scientific information for the diagnostic and therapeutic molecular and nanomedicine community and allied professions involved in the efforts of integrating molecular imaging and molecular therapy.”
As Chen explains, while other publications may discuss the separate and important roles of medical diagnostics (particularly imaging) and various forms of image-guided nano- and phototherapy, theranostics (also called “theragnostics” in some forums) encompasses the unique technologies that combine both diagnostics and therapeutics.
“Theranostics is a tall order for tiny nanoparticles,” says Chen. “Not only must those particles enable imaging in real time and then deliver a therapeutic response, they must also be safely eliminated from the body.” Chen says that the “formula” of the nanoparticle is of utmost importance: It needs to be tagged with the proper disease-seeking peptides or proteins to support visible, fluorescent, or optical molecular imaging requirements as well as drug-delivery or therapeutic capabilities.
Like optogenetics, theranostics is an emerging science less than ten years old. “Although nanoparticles are readily available for industrial and cosmetic purposes, they have a long way to go in medical applications,” adds Chen. “Fortunately, all pharmaceutical companies have imaging diagnostic centers that are driving the development of theranostics to determine the efficacy of drugs in preclinical settings. And academic institutions are furthering the effort with breakthrough discoveries.” For example, Rice University (Houston, TX) is exploring cancer-targeting gold nanoparticles that form laser-induced nanobubbles to image cancer at low light levels and burst to destroy cancer at higher illumination levels. Indeed, theranostics has arrived!
Nanoparticles that pack a punch
In addition to creating gold nanoparticles that kill cancer cells via laser-induced nanobubbles, Rice University researchers in the Laboratory for Nanophotonics (LANP) along with researchers from Baylor College of Medicine (BCM) and the University of Texas (UT) MD Anderson Cancer Center (all in Houston, TX) have created nanoparticles that kill cancer cells with heat. The researchers are the recipients of a five-year, $1.8 million grant from the National Cancer Institute’s (NCI) Alliance for Nanotechnology in Cancer program that will fund preclinical testing for theranostic treatment of pancreatic cancer—a difficult-to-treat and deadly form of cancer with a postsurgical survival rate of less than 25%.1
Invented by Naomi Halas, Stanley C. Moore Professor in Electrical and Computer Engineering and professor of chemistry and biomedical engineering at Rice University, the cancer-seeking, cancer-killing nanoparticles typically consist of a spherical silica core wrapped in a several-nanometer-thick gold shell.2 A thin dielectric layer applied over the nanoshell adsorbs near-infrared (IR) fluorophores such as indocyanine green (ICG) that are used as contrast agents for fluorescence imaging (see Fig. 1). The nanoparticles can also be seeded with active markers for magnetic resonance imaging (MRI) to track the nanoparticles throughout the body and observe their distribution within tumors before, during, and after treatment. After a tumor absorbs sufficient numbers of nanoparticles that can be tagged with antibodies to seek specific cancer types, illumination of the cancer site with near-IR (650–950 nm) light causes nanoparticle heating (nanoshells are an excellent near-IR absorber) and tumor-cell destruction.
Halas’ gold nanoshells have already proven effective in eradicating tumors in laboratory animals.3 In a 2004 animal trial with 25 mice with tumors ranging in size from 3 to 5.5 mm, the group of animals that received nanoshell injections and, six hours later, near-IR irradiation (from a 5 mm wide beam) of the skin over the tumor site had no detectable tumors (and no skin damage) two weeks after the photothermal treatment. Surface temperature measurements taken on the skin above the tumors during laser treatment showed an average temperature rise of 46 ºF.
Nanospectra Biosciences (Houston, TX), who worked with Rice University on the 2004 small-animal trial, recently demonstrated nanoparticle-directed ablation of the canine prostate in collaboration with colleagues at BCM and UT.4 Illumination of 150-nm-diameter nanoshell particles injected directly into canine prostate glands was delivered by a 15 W, 810 ± 20 nm laser (Diomed) input to a 400-µm-core optical fiber catheter terminated with a 1 cm isotropic diffuser. The catheter—with 3.0 and 3.5 W average-power output—was passed through the affected prostate in a controlled manner. In this study, the nanoshells were not designed to specifically target cancerous tumors but serve merely as a proxy for eventual cancer-seeking nanoshells that would accumulate within cancerous tissue, as the intent is to show the effectiveness of localized nanoparticle ablation. The study showed that 3 W power levels, only marginally ablative to normal tissues, cause localized heating of the near-IR absorptive nanoparticles and create radial lesions confined to within 4 mm of the optical catheter with sub-millimeter accuracy. “We have demonstrated intravenous particle delivery in numerous animal models as well as squamous cell tumors in humans,” says Donald Payne, president and CEO of Nanospectra Biosciences. “This canine study prepares us for a study in human prostate cancer,” he adds.
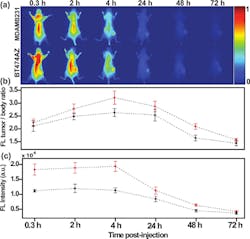
Another dramatic tumor-eradication method from researchers at the Korea Institute of Science and Technology and Kyung Hee University (both in Seoul, Korea) uses a hydrophobic photosensitizer, chlorine e6 (Ce6), chemically conjugated to human serum albumin (HSA), the most abundant protein in human blood plasma.5 The conjugates form nontoxic, self-assembled nanoparticle structures (average diameter 88 nm) in aqueous conditions. When illuminated by a 671 nm pulsed laser diode (to excite the Ce6 molecules), 600–700 nm fluorescence emission is detected using a fast photomultiplier tube (Hamamatsu) and a time-correlated single-photon counting system (Becker and Hickl GmbH). Essentially, photosensitizers are nontoxic to cells in the absence of light; however, when illuminated with a specific activating wavelength, they generate cytotoxic singlet oxygen that destroys target cells through apoptosis or necrosis. Photosensitized nanoparticles in solution penetrate the pores of newly generated vessels in tumor sites, killing the permeated cancer cells and demonstrating the theranostic efficacy of Ce6-HSA nanoparticles (see Fig. 2).“Magnetic iron oxide nanoparticles [IONP] are becoming increasingly attractive as theranostic nanoparticles due to their low toxicity, stronger enhancement of protein relaxation, and lower detection limit in comparison with traditional gadolinium-based magnetic resonance [MR] contrast agents,” says Andrew Y. Wang, president of Ocean NanoTech (Springdale, AR). “These IONPs can generate heat to kill tumor cells in the presence of compatible radio frequencies, or be used as effective drug carriers owing to their large surface-to-volume ratio.”
Ocean NanoTech provides water-soluble, biocompatible, and very stable iron oxide nanoparticles to support research needs across the United States and has several ongoing collaborations, including a project with Emory University School of Medicine (Atlanta, GA) professors Lily Yang and Hui Mao to develop IONPs that can break the barriers in hard-to-penetrate pancreatic cancer tissues and deliver multiple chemotherapeutic drugs and small molecules into the tumor cells (see Fig. 3).6, 7 One of the approaches loads the chemotherapeutic agent doxorubicin (Dox) to the IONP surface through hydrophobic interaction; each 10-nm-diameter IONP can load more than 600 Dox molecules. Another approach loads drugs to the IONP surface through pH change, heat, or enzyme application. This latter project is funded by the National Cancer Institute’s (NCI) Cancer Nanotechnology Platform Partnership (CNPP) program, which is dedicated to the advancement of new nanotechnology discoveries and their transformation into cancer-relevant applications with clinical utility.Quantum-dot theranostics
With size-tunable emission spectra and stronger fluorescence (10–100X) with higher stability against photobleaching (10–1000X) compared to fluorescent proteins or organic fluorophores, quantum dots—typically 5–50 nm in diameter—are playing a significant role in theranostic applications.8
Researchers at the University of Bucharest (Bucharest, Romania) have developed a multifunctional nanoparticle platform encapsulating hydrophilic (water soluble) quantum dots and chemotherapeutic agents.9 The drug gemcitabine, a nucleoside promoter of apoptosis (programmed cell death), is dissolved in a chitosan solution together with cadmium sulfide (CdS) quantum dots. Nanoparticles 100 nm in diameter that can be administered intravenously are obtained by processing this polymeric solution using a water-in-oil microemulsion as the nanoreactor. These nanoparticles with complex morphology contain both imaging agents and drugs encapsulated in a biocompatible polymeric shell, easily functionalized to target cancer cells. The group is also working on hydrophobic (non-water-soluble) particles to reduce potential toxicity. “So far, the particles show strong fluorescence for imaging purposes, and drug delivery and uptake functionality is being assessed,” says University of Bucharest associate professor Ludmila Otilia Cinteza. “The theragnostic concept seems to be a promising alternative to present cancer therapies. These multifunctional nanoparticles can simultaneously perform bioimaging for diagnostics, deliver drugs to the targeted tumor, and monitor the efficiency of the treatment.”
REFERENCES
1. www.media.rice.edu/media/NewsBot.asp?MODE=VIEW&ID=14919&SnID=256960677
2. R. Bardhan et al., NanoLett., 10, 12, 4920–4928 (November 2010).
3. www.media.rice.edu/media/NewsBot.asp?MODE=VIEW&ID=4469&SnID=2037091375
4. J.A. Schwartz et al., Lasers in Surgery and Medicine, 43, 213–220 (2011).
5. H. Jeong et al., Theranostics, 1, 230–239 (2011).
6. www.surgery.emory.edu/news-and-events/news-reports/yang_NIH_NCI_nano_grant_2010.html
7. http://oceannanotech.com/show_news.php?nid=43
8. Y.-P. Ho and K.W. Leong, Nanoscale, 2, 60–68 (2010).
9. L. O. Cinteza, “Multifunctional nanosystems for cancer theragnostics,” SPIE Newsroom online; doi: 10.1117/2.1201103.003432 (Apr. 20, 2011).

Gail Overton | Senior Editor (2004-2020)
Gail has more than 30 years of engineering, marketing, product management, and editorial experience in the photonics and optical communications industry. Before joining the staff at Laser Focus World in 2004, she held many product management and product marketing roles in the fiber-optics industry, most notably at Hughes (El Segundo, CA), GTE Labs (Waltham, MA), Corning (Corning, NY), Photon Kinetics (Beaverton, OR), and Newport Corporation (Irvine, CA). During her marketing career, Gail published articles in WDM Solutions and Sensors magazine and traveled internationally to conduct product and sales training. Gail received her BS degree in physics, with an emphasis in optics, from San Diego State University in San Diego, CA in May 1986.