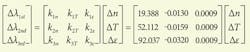FIBERS FOR SENSING: Label-free FBG-based biosensor integrates microfluidic channels
SIMARJEET S. SAINI, SANG-MAE LEE, MYUNG-YUNG JEONG, and MARIO DAGENAIS
Optical biosensors promise major advances in medical diagnostics, environmental monitoring, drug development and quality control, and homeland security. They can broadly be characterized into two categories: fluorescence-based sensors and label-free sensors.
For fluorescence-based sensors, the target molecules are tagged with fluorescent dyes and the fluorescence spectrum is detected in presence of the targeted molecules. This allows for extremely sensitive detection down to the single-molecule level; however, quantitative measurements are difficult, the process is laborious, and the tags may affect the function of the biomolecules.1
In contrast, label-free detectors detect targeted molecules in their natural form by attaching those molecules to probe molecules on the surface of the sensor and measuring the change in the optical properties of the sensor. Integrated optics based on planar lightwave circuits like ring resonators or surface plasmonics, and fiber-optic-based sensors have been proposed and demonstrated over the last few years.
While these integrated-optics-based sensors offer attractive features such as the possibility to integrate multiple sensors on a single chip, they suffer from costly micro-optic coupling components and resultant high packaging costs. But fiber-optic sensors offer distinct advantages as biochemical sensors due to their ease of optical coupling, small weight and low cost, ability to be used for distributed sensing through fiber networks and multiplexing of different sensors by wavelength-division multiplexing, and chemical and biological inertness due to the fiber-optic glass material itself.2, 3
The FBG advantage
For fiber Bragg grating (FBG)-based sensors, the sensing capability is directly encoded into the wavelength of the reflected Bragg mode, making it an absolute measurement.4 The surface of the fiber is sensitized with a probe molecule with selective attachment to the target. If the target molecule is attached, it changes the refractive index on the surface of the sensor, resulting in a change in the effective index of the fiber mode and a measurable shift in the reflected Bragg wavelength peak.
Our research team has increased the sensitivity of FBG-based biosensors by etching into the core of the optical fiber. Furthermore, we have integrated the sensor with bio-compatible polydimethylsiloxane (PDMS) microfluidic channels.5 By measuring three optical modes at the same time, we can sense changes in refractive index, temperature, and strain simultaneously, allowing in situ measurements of chemical reactions. To our knowledge, this is the only demonstrated sensor with the ability to measure these three parameters in a single, simultaneous measurement.
Integrated microfluidics
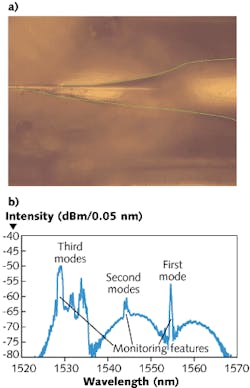
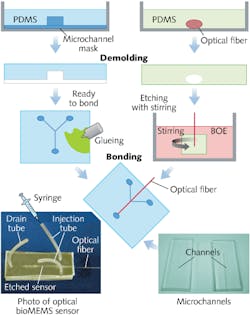
The PDMS material has the unique property of being optically transparent, and PDMS-to-PDMS bonding allows fabrication of simple sensor structures with low production cost (see Fig. 1). A hard mask is used to microimprint a microfluidic channel onto the PDMS structure. We used a channel width of 210 µm for ease of placement of the unetched part of the FBG. Channel height was 50 µm and length was 1.2 cm.Our sensor was etched to a diameter of 7 µm. At this diameter and assuming water as the surrounding medium, the fiber core can support up to ten linearly polarized modes as a cylindrical waveguide. The higher-order modes have an increased evanescent field compared to the fundamental mode, resulting in an increased sensitivity to surrounding refractive-index changes.
When the temperature is changed, the refractive index of the core also changes. Since the fundamental mode is more confined in the core of the waveguide, the behavioral change to the change of temperature is different for different modes as compared to that of the change of surrounding refractive index. When strain is applied to the fiber, it basically changes the grating period and results in a change in Bragg wavelength for all the modes.
Therefore, by exciting and measuring at least three fiber modes at the same time, one can differentiate between the different parameters simultaneously. Excitation of these modes with adequate signal-to-noise ratio requires a careful design of the transition region between the unetched and the etched part of the fiber.
For our sensor, this was achieved by using an asymmetrical taper: the width varies linearly on one side and exponentially on the other (see Fig. 2). By measuring the reflected spectrum in situ, we can change the etching parameters to achieve high yield in exciting the three modes. Furthermore, by measuring the wavelength of the reflected peak, the final diameter can be controlled to an accuracy of ±0.1 µm.
Sensor performance
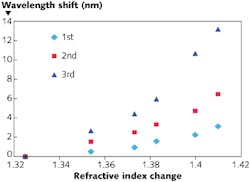
To evaluate sensor performance, the sensor was immersed in Cargille Laboratories (Cedar Grove, NJ) fluids with different refractive indices (see Fig. 3). The sensitivity for the higher-order modes is larger and is equal to 19.4, 52.1, and 92.0 nm/riu (refractive-index unit) for fundamental, second order, and third order modes, respectively, at refractive indices close to water (1.325). The sensitivity increases as the refractive index is increased because of reduced guidance in the optical modes.For 1 pm resolution in measuring changes in the Bragg reflections, an index resolution of 1 × 10-5, a strain resolution of 1 microstrain, and a temperature resolution of 0.032ºC was achieved. The refractive-index sensitivity achieved is better than the 70 nm/riu achieved in planar-lightwave-circuit-based ring resonators.6
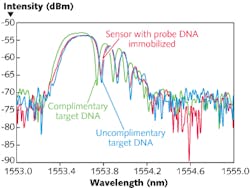
The sensor was also used to characterize hybridization of DNA. Single-stranded DNA oligonucleotide probes of 20 bases were immobilized on the surface of the fiber grating using relatively common glutaraldehyde chemistry. Hybridization of complementary target single-strand DNA oligonucleotide was monitored in situ and successfully detected (see Fig. 4).The high sensitivity of this biosensor can be utilized to measure a variety of biologically relevant processes including DNA-DNA or DNA-RNA hybridization, protein-protein interactions, and carbohydrate-protein interactions under physiological conditions. By measuring refractive index, temperature, and strain simultaneously, experimental variations can be easily corrected.
REFERENCES
1. W.E. Moerner, Proc. Natl. Acad. Sci., 104, 12596–12602 (2007).
2. Y. Sano et al., IEEE J.Lightwave Technol., 21, 1, 132–139 (January 2003).
3. B. Culshaw, IEEE J. Lightwave Technol., 22, 1, 39–46 (January 2004).
4. A.D. Kersey, Optical Fiber Technol., 2, 291–317 (1996).
5. O.C. Jeong et al., Sensors and Actuators A, 135, 849–856 (2007).
6. K.D. Vos, et al., Opt. Expr., 15, 7610–7615 (2007).
7. C. Stanford et al., Current Analyt. Chem., 4, 4, 356–361 (2008).
8. G. Ryu et al., IEEE J. Selected Topics in Quant. Electron., 16, 3, 647–653 (2010).
Simarjeet S. Saini is assistant professor at the University of Waterloo, 200 University Ave. West, Waterloo, ON, Canada N2L 3G1; e-mail: [email protected]; http://photonics.uwaterloo.ca. Sang-Mae Lee is assistant professor and Myung-Yung Jeong is professor and director of the Department of Cogno-Mechatronics Engineering at Pusan National University, 30 Jangjeon-dong, Geumjeong-gu, Busan 609-735 Korea; http://english.pusan.ac.kr. Mario Dagenais is a professor at the University of Maryland, Electrical & Computer Engineering Department, Kim Building 2128, College Park, MD 20742, USA; www.ece.umd.edu/photonics.
The microfluidic channel includes a Y-junction consisting of two feed channels and one drain channel. The optical fiber was partly stripped of its acrylic coating and then molded into another PDMS substrate. The ends of the fiber were epoxied in place on the PDMS substrate and the fiber was etched in buffered oxide etch (BOE) to achieve an asymmetrical taper. The PDMS holder was kept in a BOE bath stirred with a magnetic stirrer to create a laminar flow. By adjusting the position of the holder laterally in the bath and the temperature of the bath and the stirring speed, one can control the nature of the taper.
After the etch, the quality, Q, of the FBG response decreases due to increased losses, and the spectral width broadens with respect to the unetched FBG. As such, for monitoring purposes, the filter edge of the sensor is measured instead of the peak. This is done by locally thresholding the response and fitting a straight line along the edge. The spectrum is measured at a resolution of 10 pm. By doing the linear fit on the edge, a variation of 1 pm in the edge is detectable.
This sensitivity increase can be observed through the nonlinear response of the wavelength shift to the change in the refractive index. The sensor response due to change in refractive index, temperature (change of core refractive index), and strain can be summarized in matrix form:
Because in situ measurements can be made, different chemical reactions and their time evolutions can be studied. For example, it was shown that 3-aminopropyl-monoethoxydimethyl-silane creates a monolayer attachment on top of the glass surface, whereas 3-aminopropyl-triethoxysilane creates a polymer chain up to five molecules deep. Furthermore, during the silanization process, an adsorbed water layer is created on the surface of the sensor.7 Similarly, the sensor has also demonstrated the covalent attachment of carbohydrate derivatives tested by binding of high-specificity lectins, paving the way for studies of human glycome—sugars (carbohydrates) in the human organism.8 This property of the sensor can be used to understand and optimize various chemical reactions.