Femtosecond X-ray diffractive crystallography could enable damage-free molecular imaging
Tempe, AZ--"From the beginning, the resolution of images recorded by biologists has been limited by damage due to the radiation used," said physicist John C.H. Spence, a Regents' Professor in Arizona State University's (ASU's) College of Liberal Arts and Sciences, one of the lead authors of a new study published February 3 in Nature that details a new method to determine structures of biomolecules based on diffraction from protein nanocrystals delivered by a tiny aerojet nozzle into an X-ray beam. "But what happens if a pulse of imaging radiation is used that terminates before damage begins, yet contains sufficient photons to generate a useful scattering pattern?"
Based on nanocrystallography experiments that took place in December 2009 at SLAC in California, ASU supplied nanocrystals of photosystem I, a large membrane protein complex consisting of more than 100,000 atoms that acts as a biosolar energy converter in the process of oxygenic photosynthesis. R. Bruce Doak, an ASU physics professor who has more than 30 years experience in the science of supersonic atomic and molecular beams, said of the experiment, "The ASU liquid jet is performing flawlessly. We put together a dual reservoir supply system that allows us to switch samples without ever turning off the flow, and so the nozzle has now been running continuously for over 50 hours."
"Early on, we established the sample concentration of microcystallite photosystem I that delivers an X-ray diffraction pattern with almost every X-ray pulse--30 diffraction images per second! No one here had ever seen anything like a 100% hit rate before, much less sustained for days; over 3 million stored diffraction images so far. The diffraction patterns are gorgeous," wrote Doak, also an author on the Nature nanocrystallography paper.
"These are early days for femtosecond diffractive imaging," noted Spence, who provided the theory and much of the data analysis. "But first indications are that high-resolution data can now be obtained at the nanoscale by this method. If we can indeed 'outrun' the many radiation-damage processes in this way, it will open the way to future experiments on laser-excited samples, 3-D image reconstruction and a host of other experiments on fast imaging, all directed to the grand challenge of obtaining movies showing molecules at work."
The ASU work is part of a new $1.46 million grant from the National Institutes of Health to further study femtosecond nanocrystallography of membrane proteins. "The aim of the grant is to develop the new method further, which may well revolutionize the field of structural biology. That said, the innovations developed for this grant will surely produce exciting and radically new research activities," said William Petuskey, chair of ASU's Department of Chemistry and Biochemistry.
SOURCE: Arizona State University; https://asunews.asu.edu/20110202_nanocrystallography
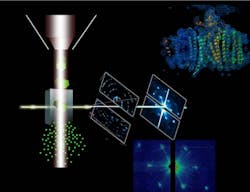
IMAGE: In femtosecond diffractive nanocrystallography, the ASU protein beam injector, left, ejects nanocrystals in a fully hydrated stream of mother liquor. The stream of crystals interacts with the LCLS free-electron laser X-ray beam, where the crystals instantly explode. The free-electron laser X-ray beam destroys any solid material in its focus, forming a plasma that reaches temperatures higher than the inside of the sun. However, each laser pulse is so short that diffraction can be detected from each nanocrystal before it is destroyed. Data evaluation of just 10,000 diffraction patterns reveals the structure of the protein at molecular resolution, shown in the top right of this image. (Image by Petra Fromme/Arizona State University)