Recognizing mirror images of molecules with a femtosecond laser
Kassel, Germany--A compact femtosecond-laser system developed by a group at the University of Kassel distinguishes certain molecules from their mirror images —knowledge that is crucial when one version of the molecule is a drug and its mirror image is toxic.1
The technique is an improvement on a previous technique in which synchrotron radiation (highly energetic photons from a particle accelerator) is used to eject electrons from the molecules with the electrons' trajectories analyzed to provide the required info.
In the University of Kassel approach, the individual high-energy photon is replaced by three laser photons that excite the molecule through intermediate levels until it releases an electron; this method is known as resonance-enhanced multiphoton ionization (REMPI). “It is thus possible to eject electrons with less energetic but more intense light,” says Thomas Baumert, the lead researcher.
For the measurements, the light must be circularly polarized. Molecules in the gas phase are randomly oriented and thus encounter the laser light from all possible angles; the ejected electrons also fly off in every possible direction as they leave the molecule. By careful measurement and calculation, the team is able to determine the distribution of the angles of the electrons’ flight paths.
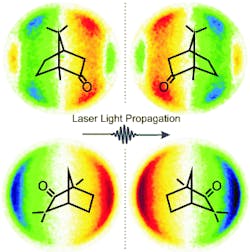
In the case of linearly polarized light, the distribution is symmetrical. “However, when the electrons are ejected by circularly polarized light, we find a distinct asymmetry to the angles at which the free electrons are found in relation to the laser beam,” says Baumert. “This asymmetry is inverted if left circularly polarized light is used instead of right, an effect known as photoelectron circular dichroism. We observe the same effect when we keep the circular polarization the same but change from the right-handed to the left-handed structure of the chiral molecule being observed.” The researchers were able to demonstrate this with the chiral compounds camphor and fenchone.
REFERENCE:
1. Christian Lux et al., Angewandte Chemie, first published online 20 February 2012; DOI: 10.1002/anie.201109035

John Wallace | Senior Technical Editor (1998-2022)
John Wallace was with Laser Focus World for nearly 25 years, retiring in late June 2022. He obtained a bachelor's degree in mechanical engineering and physics at Rutgers University and a master's in optical engineering at the University of Rochester. Before becoming an editor, John worked as an engineer at RCA, Exxon, Eastman Kodak, and GCA Corporation.