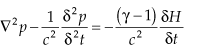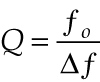Optical-nose technology competes with breathalyzers and blood tests
To many, the term "breathalyzer" conjures up images of flashing red lights, sobriety checkpoints, judges, and jail. But to someone involved with dialysis, either as a patient or practitioner, the term may soon connote rapid diagnosis, improved results, decreased cost, improved quality of life, and extended life span.
The lung-blood barrier is transparent to many small molecules. When we inhale, oxygen passes through the lung directly into the bloodstream, and when we exhale, carbon dioxide is expelled. In addition to these gases, expired breath contains a rich mixture of many other gases at parts-per-billion (ppb) levels, which not only can be quantitatively correlated with the amounts of the same gases in the bloodstream, but with concentrations of other molecules as well.
The presence of trace amounts of ammonia, nitric oxide, aldehydes, and ketones in the expired breath has been linked to kidney/liver malfunction, asthma, diabetes, cancer, and ulcers. The presence of carbon disulfide, ethane, butane, and pentane has been linked to neurological disorders. Consequently, the ability to rapidly, accurately, and noninvasively determine the level of these gases in a patient's breath in situ and in real time can have a profound impact on diagnosis, treatment, and end results. For example, during dialysis, monitoring the level of ammonia in the breath of a patient with renal failure can determine the level of blood urea nitrogen (BUN) at the start of and during the process, and can accurately indicate when the cleansing process is complete.
Pranalytica has developed ultrasensitive, laser-based, trace-gas detection technology (named O-Nose for optical nose) and instrumentation to enable noninvasive, real-time, and cost-effective diagnosis of a variety of vital human physiological functions. In addition to medical applications, the technology can be used in an industrial environment to monitor ambient air for the presence of contaminating, toxic, and explosive substances.
Detection technology
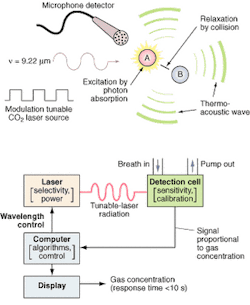
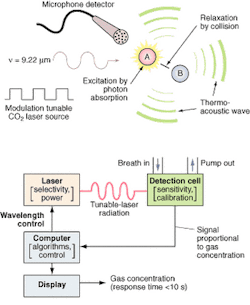
FIGURE 1. The photoacoustic process (top) is implemented by the O-Nose platform (bottom) in which the computer determines the appropriate wavelength for the target species and adjusts the laser to the appropriate spectral line. The patient breathes into a mask or tube connected to the calibrated acoustic detection cell, and the microphone detector picks up and amplifies the acoustic pressure wave generated by the relaxing molecules. The output signal is directly proportional to the concentration of the target species in the laser beam. The signal is processed by the computer and the results are displayed on the screen. Response time is less than 60 seconds, basically the time it takes for the breath to pass through the inlet tube into the measurement chamber.
The detection technology uses photoacoustic spectroscopy, an absorption-based diagnostic tool, to detect and measure ppb concentrations of trace gases in expired breath. In brief, photoacoustic spectroscopy involves four steps (see Fig. 1): modulating the laser radiation, either in frequency or amplitude, at a wavelength that overlaps with a spectral feature of the target molecule; exciting the target molecule by absorption of the incident radiation; deactivating the excited molecule via collisions, during which the absorbed radiation energy is converted into periodic local heating at the modulation frequency; and monitoring the resulting acoustic waves with a microphone.
The conversion of heat input from incident laser radiation into acoustic waves is described by the wave equation:
null
where p is the pressure, H is the generated heat, and γ is the ratio of specific heats. When the heat input is constant, δH/δt = 0, no pressure wave is generated. Thus, the heat must be modulated, which requires that the laser radiation must be modulated, either in amplitude or frequency. The magnitude of the photoacoustic signal that results is described by
null
where C is a cell-specific constant, P is the incident laser power, N is the number density of absorbing molecules, σ is the absorption cross section of the transition that is being interrogated, Δt is the cycle period of the modulated radiation, and Sm is the sensitivity of the microphone. The Δt term indicates that, for nonresonant operation, the signal decreases with increasing modulation frequency. As a result, most nonresonant measurements are made with modulation frequencies between 10 and 100 Hz.
Unfortunately, 1/ƒ noise is also higher in this measurement regime. The cell-specific constant C is a function of cell geometry, measurement conditions, and the modulation frequency. For measurement cells that are used as acoustic resonators, signals at resonance are significantly larger than those for nonresonant operation, despite the inverse dependence from the Δt term. The amount of signal enhancement that occurs when the laser is modulated at a resonant frequency is determined by the quality factor Q, which is the resonant frequency ƒ0 normalized by the half width of the resonance profile, Δƒ:
null
It is clear from the last two equations that doubling the laser power, the number of molecules of the target species in the optical path, the microphone sensitivity, or, in the case of near-resonant operation, the cavity Q doubles the output signal.
Stable, sealed CO2 lasers
Grating-tuned carbon dioxide (CO2) lasers have long been used in the laboratory for photoacoustic trace-gas detection because of their high output power and their ability to produce discrete wavelengths that are strongly absorbed by species such as ammonia, benzene, and ethylene. They operate at approximately 120 discrete wavelengths between 9 and 11 µm. The CO2 laser is particularly useful for detecting ammonia because one of its laser lines near 9.22 µm is nearly coincident with one of ammonia's strongest spectral feature, the sR(5,K) multiplet. Despite these advantages, CO2 lasers have not typically been incorporated into commercial gas-sensing equipment because of their size, power requirements, and lack of long-term stability when operating on a single spectral line. This situation has changed with the availability of sealed-off, low-power (<25 W) grating-tunable CO2 lasers with excellent spectral resolution and stability as well as a compact footprint that can be easily deployed in a medical or industrial package.The Pranalytica platform uses a standard Coherent-DEOS CO2 laser that has been modified to incorporate a software-tunable custom grating. The laser has undergone a wide variety of performance and reliability tests, including repeated cycling between spectral lines every 10 seconds for 9000 hours. In the system, the laser can operate without adjustment for more than 15,000 hours.
The first laser breathalyzer
The O-Nose technology has been used to construct an instrument that measures ppb-level ammonia in expired human breath in the presence of a large number of other interfering species including >4% carbon dioxide and >10% water vapor. This instrument has been deployed in the kidney dialysis center at the University of California, Los Angeles, and in the obstetrics/gynecology center in the Olive View Medical Clinic in Sylmar, CA (see Fig. 2). Physicians and nurse practitioners at these sites use the instruments to collect clinical data on kidney dialysis patients and potential pre-eclampsia patients. Pre-eclampsia affects about 5% of pregnant women in their third trimester.
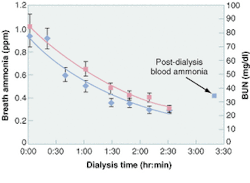
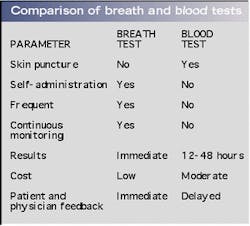
An ongoing clinical study correlates breath ammonia measurements for kidney dialysis with simultaneously determined BUN and creatinine data obtained through taking blood samples (see Fig. 3). The advantages of breath tests over blood tests are numerous (see table, right). The breath-ammonia measurement protocol will provide the physician and the dialysis center staff with the real-time feedback needed to improve the quality of care given to the patient. In a home-dialysis environment, the technology promises the ability to assess simply the progress of dialysis and indicate to the patient when to stop a treatment session. This capability goes a long way toward ameliorating many of the serious quality-of-care issues voiced at the recent hearings by the U.S. Senate Subcommittee on Aging, and will provide an objective measure of the efficacy of the dialysis treatment.
Broad applications
The technology used for trace-gas detection is not limited to medical applications. Pranalytica recently introduced a trace-ammonia analyzer for industrial applications with a noise-limited detection sensitivity of ±0.2 ppb and an integration time of less than 30 seconds. The analyzer is destined for use in deep-UV submicron lithography applications where ambient ammonia concentration at levels as low as 10 ppb can be detrimental to the photoresists and impair the quality of the final product. The detection of this contaminant alone can save the foundries millions of dollars a year.
Another analyzer has been designed to detect gases other than ammonia. We are currently determining minimum sensitivity levels for a wide variety of industrial gases including hydrogen cyanide (<4 ppb), acetylene (<3 ppb), carbon monoxide (<650 ppb), and carbon dioxide (<55 ppm). Sub-ppb trace-gas analysis can also be used to detect explosives and chemical warfare agents such as nerve gases. This capability has immediate relevance to homeland security and battlefield applications.
In the semiconductor industry, the technology will have a significant role in maintaining the clean-room environment needed for the next generation of deep-UV lithography applications. And in the medical arena, noninvasive diagnostics with real-time point of care availability of the results is made possible by the marriage of photoacoustic spectroscopy to long-lived, stable, sealed, grating-tuned CO2 lasers. This is likely to have a significant impact on health-care delivery and cost containment.
C. KUMAR N. PATEL is a professor of physics at UCLA and chairman of the board at Pranalytica, 1101 Colorado Blvd., Santa Monica, CA 90401; e-mail: [email protected]. ERIC MUELLER is manager of electro-optic engineering and systems at Coherent-DEOS, 1280 Blue Hills Ave., Bloomfield, CT 06002; e-mail: [email protected].