Drawing strongly on rapid technological developments fueled by massive investments in optical telecommunications, biomedical optical-imaging technology is achieving unprecedented spatial resolution (on the order of a few microns in the case of optical coherence tomography), centimeters of subsurface penetration using near-infrared wavelengths in the case of diffuse optical tomography, as well as wider ranges of functional information based on a variety of methods that include Doppler techniques and the use of fluorescent and phosphorescent markers.
"The hot areas in biomedical optical imaging include fluorescence/phosphorescence imaging, multiphoton microscopy, fluorescence lifetime imaging, optical coherence tomography, and diffuse optical tomography," according to Sergio Fantini, a biomedical engineering professor at Tufts University (Medford, MA) and moderator of the Biomedical Optics Hot Topics session at Photonics West (San Jose, CA) in January. "Among the emerging areas that show significant potential one could mention the synergistic combination of light and ultrasound in optoacoustic tomography and ultrasonically modulated optical tomography."
Fluorescence/phosphorescence imaging, multiphoton microscopy, and fluorescence lifetime imaging all measure the spatial distribution of endogenous fluorophores in the sample, or of exogenous fluorescent/phosphorescent molecules used as an optical label, Fantini said. "For example, the use of phosphorescent-sensitive probes allows for spatially resolved measurements of the oxygen tension using phosphorescence lifetime imaging, and molecular imaging uses fluorescent probes to optically label cancerous tissue."
Two-photon microscopy excites the fluorescence only in the focal spot of illumination, thus allowing for z-discrimination and achieving spatial confinement of photodamage to the sample. Fluorescence lifetime imaging is based on time-resolved optical techniques either in the time domain or frequency domain. Optical coherence tomography is an interferometric, backscattering imaging technique that provides cross-sectional images of tissue. Diffuse optical tomography relies on the multiply scattered light in tissue. As a result, diffuse optical tomography achieves limited spatial resolution, but can noninvasively probe tissues such as the human brain and human breast.
Optoacoustic tomography uses the localized optical absorption of areas of interest (such as tumors) to induce pressure waves that are detected with ultrasonic transducers. Ultrasonically modulated optical tomography uses a focused ultrasound beam to modulate the intensity of the detected speckle pattern with a modulation depth that depends on the optical properties at the ultrasound focus.
Boundaries and barriers
Development of biomedical optical-imaging technologies is also highly interdisciplinary, often being described as occurring at the intersections of many different scientific and engineering disciplines (see Fig. 1 and "Fibered confocal microscopy becomes practical"). While this is true of much of optical technology, including the development of the laser itself, there is also a strong push in the biomedical-optical-imaging scientific community to move the instrumentation from the bench to the bedside, which accounts for the formation of many collaborative teams between engineering, basic scientists, and medical doctors.
"I see this as a necessary condition to advance the field of biomedical optical imaging, and such interdisciplinary flavor is evident at the scientific conferences in the field," Fantini said. "Of course, there are many other new technologies that are intrinsically interdisciplinary, but I like to think that in many areas of biomedical optical imaging, the various disciplines that contribute are equally important to guarantee a successful outcome of the research."
Successful research outcomes also must deal with the strong scattering of light that takes place within most biological tissues, which generally requires a compromise between depth of penetration and spatial resolution. "For instance, in optical coherence tomography the axial spatial resolution can be as good as a few microns, but the penetration depth is limited to hundreds of microns," Fantini said. "By contrast, diffuse optical tomography may probe tissues to a depth of several centimeters, but the spatial resolution of such deep structures is in the order of several millimeters at best."
Optical coherence tomography
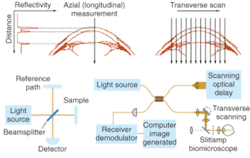
Optical coherence tomography (OCT) is essentially an optical analogue to ultrasound imaging, which is based on time delay and magnitude, according to OCT pioneer James Fujimoto, a professor of electrical engineering and computer science at the Massachusetts Institute of Technology (Cambridge, MA) and cochair of the Biomedical Optics symposium at Photonics West (January 2004; San Jose, CA). The difference, of course is that OCT measures light instead of sound, and therefore relies on interferometry because the velocity of light is too high for direct electronic detection (see Fig. 2).1In the laboratory, spatial resolution has been achieved on the order of 1 to 5 µm using short-pulse laser light sources, while commercial systems currently deliver spatial resolution on the order of 10 to 15 µm using superluminescent diode sources (see "OCT continues transition from research lab to medical market," below). The ability to provide resolutions approaching that of standard histopathology (the medical "gold standard" for examining tissue pathology), and the ability to do so in real time and in situ, are fundamental strengths of OCT for medical imaging. In addition, the development of fiberoptic technology for telecommunications is contributing to the use of OCT in existing noninvasive or minimally invasive delivery systems that include microscopes, hand-held probes, endoscopes, catheters, laparoscopes, and needles.
Three areas of particular emphasis for OCT are in: examination of tissue in organs such as the eye and brain where conventional biopsy is not feasible; helping to guide standard biopsy procedures to increase sampling accuracy and eliminate false negatives; and guiding surgical procedures, including minimally invasive surgeries or interventional procedures, particularly within the cardiovascular system.
In addition to development of ultra-high-resolution OCT using femtosecond laser sources, emerging developments in OCT technology include optical coherence microscopy (OCM), and functional OCT imaging, according to Fujimoto. OCM combines OCT coherence-gated detection with high-numerical-aperture confocal microscopy and may ultimately enable cellular imaging through an endoscope (see "Dual approach measures health of engineered tissue"). Functional OCT modalities include:
- Spectroscopic imaging, which enhances tissue contrast and enables quantitative imaging of metabolic indicators, such as tissue hydration or oxygenation of hemoglobin in blood vessels.
- Polarization-sensitive OCT, which measures tissue birefringence, enabling assessment of biological states and processes such as osteoarthritic changes, burn depth and fiber layer thicknesses.
- Optical Doppler tomography, which provides simultaneous tomographic imaging of tissue microstructure and blood flow, and also shows promise for quantitative assessment of capillary density and angiogenesis.
Diffuse optical imaging
To get beyond the depth-of-penetration limitations of OCT, diffuse optical imaging (also known as photon-migration imaging) takes advantage of a "spectral window" in human tissue from about 700 to 900 nm in which light absorption due to chromophores such as blood and water is relatively weak. Scattering effects remain strong within this window, however, generally causing light to "diffuse" in a manner that can be modeled, in the brain and breast for instance, by Monte Carlo representations of the diffusion equation and by analytic expressions (see Fig. 3).2, 3, 4Near-IR radiation enables visualization of the oxygen state of hemoglobin as well as the amount of blood flowing through muscle, brain, and breast tissue, according to optical-imaging pioneer Britton Chance, currently an emeritus faculty member at the University of Pennsylvania (Philadelphia, PA). "Blood is our main signal," he said. "There are other signals, like water and lipid, that are not really functional. This is functional near-IR imaging, however, because the blood responds to function."
Optical methods for imaging blood flow include speckle imaging and Doppler. The future of cancer detection, however, lies in molecular beacons, according to Chance. "They will revolutionize the detection of cancer," he said. The main molecule, a tricarbo cyanine that fluoresces in the near-IR, is attached to a peptide, which seeks out the genetic expression in a tumor. The entire structure is injected into the body and returns a fluorescent signal when tumors are found.
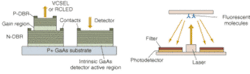
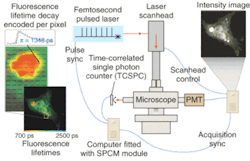
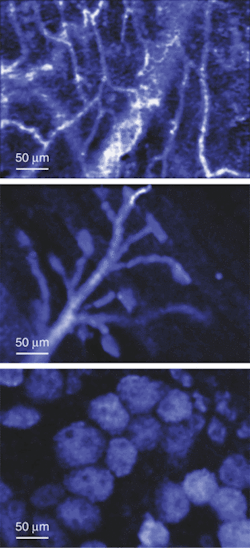
The potential benefits are not limited to cancer, of course. Neurology professor Brian Bacskai and colleagues at the Massachusetts General Hospital (Boston, MA) and the University of Pittsburgh School of Medicine (Pittsburgh, PA) are using a fluorescence lifetime-imaging microscopy (FLIM) device in conjunction with a laser-scanning multiphoton microscope (both commercially available) to validate Pittsburgh compound B (PIB) in animal models as a biomarker for the amyloid-b deposits associated with the onset of Alzheimer's disease (see Fig. 4).5, 6 Data obtained through optical bioimaging helped to validate the biomarker for use in a positron-emission tomography scanner, where it is currently undergoing clinical trials with human subjects. Even though there is currently no treatment for Alzheimer's the existence of a biomarker is expected to facilitate research that may eventually lead to a treatment.To take even further advantage of the possibilities of diffuse imaging with biomarkers, Harvard Medical School (Boston, MA) professor Vasilis Ntziachristos and coworkers at Massachusetts General Hospital have developed a volumetric imaging technique called fluorescence molecular tomography (FMT) to account for the diffusive propagation of photons in tissues (see Fig. 5). While diffuse-tomography bioimaging systems generally use a limited number of sources and detectors, the FMT system consists of a parallel plate-imaging chamber and a lens-coupled CCD camera, enabling conventional planar imaging as well as fluorescence tomography. The researchers have reported obtaining a dataset containing more than 106 measurements, superimposing anatomical features with tomographic results for improved visualization. Fluorescence molecular tomography effectively combines tomographic principles of diffraction tomography with absorption and fluorescence measurements for accurate three-dimensional (3-D) reconstruction of fluorescent marker distribution.7, 8, 9
"The tomographic imaging method offers a range of new capabilities for studying biological function, for example identifying molecular expression patterns by multispectral imaging or continuously monitoring the efficacy of therapeutic drugs," Ntziachristos said. "Developing fluorescent molecular tomography (FMT) can improve the current state of the art in imaging based in 'photographic methods' by offering three-dimensional imaging and resolving depth and multiple overlying lesions, offering accurate quantification capacity, and improving contrast while maintaining highly specific targeting of molecular events in vivo."
REFERENCES
- J. G. Fujimoto. Nature Biotechnology 11(21) 1361 (November 2003).
- A. G. Yodh et al., www.lrsm.upenn.edu/pmi/nonflash-ver/index2.html
- B. Chance, E. Anday, S. Nioka et al., Optics Express 2(10) 411 (May 11, 1998).
- S. Fantini et al., Optics & Photonics News 14(11) 24 (Nov. 2003).
- B. J. Bacskai et al., J. Biomed. Optics 8(3) 368 (July 2003).
- B. J. Bacskai, G. A. Hickey, J. Skoch et al., PNAS 100(21) 12462 (Oct. 14, 2003).
- E. E. Graves et al., Med Phys. 30(5) 901 (May 2003).
- V. Ntziachristos et al., Nature Medicine 8(7) 757 (July 2002).
- R. Weissleder, V. Ntziachristos, Nature Med. 9(1) 123 (January 2003).
Fibered confocal microscopy becomes practical
Endoscopy is an established technique for examining the interior of a living person or animal. A typical endoscopic probe, however, does not resolve at a cellular level. Also, it cannot see below the surface of the tissue it is examining. A version of confocal microscopy in which light is sent and returned through an optical-fiber bundle can push beyond both of these limitations. Engineers at Mauna Kea Technologies (Paris, France) have taken this concept and made it commercially practical, combining the disciplines of optics, high-speed electronics, and image processing.
Fibered confocal microscopy allows in-vivo imaging of microstructures such as cells and microvessels, and excels in the study of tumor physiology and angiogenesis. It can operate in either reflectance or fluorescence. As a fluorescence system, it relies either on fluorophores that reside naturally in the cells, such as GFP (green fluorescence protein) or YFP (yellow fluorescence protein), or introduced fluorophores, and it can even exploit quantum dots or fluorescent microspheres. The Mauna Kea instrument can observe below a tissue surface to a depth of up to 100 µm at a lateral resolution of 2.5 to 5 µm, an axial resolution of 15 to 20 µm, and a 400-µm field of view (see figure).In the instrument, laser light at 488 nm is modulated to produce a continuous string of pulses 100 ns apart. A fiber bundle consisting of 30,000 optical fibers—each less than 2 µm in diameter—is terminated at its probe end by an objective-lens assembly that can have a diameter anywhere from 1.8 mm down to 350 µm. At the other end of the bundle, an optical system containing two orthogonal scanners directs a focused 2-µm spot across the fiber-bundle endface in a fine raster pattern. In fact, the scan is so fine that it crosses each fiber several times, which in practice boosts signal contrast. A beamsplitter sends the return light to a detector. The pulses returning from the tissue under examination arrive 30 ns later than the input pulses depart, making possible time multiplexing to further increase contrast, image averaging improves the signal-to-noise ratio.
Through image reconstruction and processing, the fiber-bundle geometry is eliminated from the image. Histograms and other statistics (median, standard deviation, and so on) can be calculated from the image. More-complex calculations can be performed. For example, the outlines of cells can be automatically determined and their number, mean diameters, and mean cross-sectional areas can be calculated.
The study of tumors relies heavily on the use of animal testing, the minute diameters of the probes of the Mauna Kea instrument allow researchers to investigate even small animals. "Endoscopic access to hollow organs is possible even in mice," says Sacha Loiseau, chief executive officer and cofounder of Mauna Kea Technologies. "Access with a small incision also enables the observation of intact organs deep inside the body of the animal." He notes that no other technology allows microscopic examination of animals in such a manner without killing them.
"As for clinical applications, looking into hollow organs (with either a fluorescence system or a reflectance system) is, of course, the prime application," says Loiseau. "The idea is to use the endoscopy vector (a regular videoscope) to place a probe on a region that has been tagged as suspect by a wide-field instrument such as a chromoendoscope or an autofluorescence endoscope. A specific diagnosis may then be obtained in situ, which will greatly help to decide if any action has to be taken—and, if so, which one (such as taking a biopsy) at the right location. We are currently in a clinical-trial phase for these applications."—John Wallace
OCT continues transition from research lab to medical market
Carl Zeiss Meditec (Jena, Germany) has developed prototype OCT systems for imaging the anterior chamber of the eye in collaboration with Dr. David Huang and colleagues at the Cleveland Clinic (Cleveland, OH). Retinal imaging systems, which are becoming the standard of care in ophthalmology, image at 820 nm to penetrate the water within the eyeball and at powers low enough to be safe for the sensitive retinal tissue, while the anterior chamber imagers use more powerful 1300-nm light sources for rapid imaging of anterior chamber features such as the iris and cornea (see figure)."Probably the biggest potential application that will affect a lot of people is Phakic intraocular lenses (IOLs)," said Matt Everett, senior program manager for optical imaging. In Europe in particular, the placement of IOLs has been gaining favor as an alternative to surgical procedures to reshape the cornea. Simply placing a lens behind the cornea significantly reduces the tissue damage and subsequent healing associated with the procedure. Successful placement of a Phakic IOL requires precise measurement of the anterior chamber, however, which can be obtained through anterior-chamber imaging. Anterior chamber imaging, which also has possible applications in glaucoma and LASIK procedures, Everett said.
At the same time, LightLab Imaging (Westford, MA), whose cardiovascular OCT imagers are now undergoing clinical trials in Japan, is also collaborating with Asahi Pentax (Orangeburg, NY) and Lantis Laser (Hewitt, NJ) to develop OCT systems for gastrointestinal and dental applications, respectively. A major focus of the work at LightLab has been to develop clinical OCT systems using source and fiber technology originally developed for telecommunications applications. "Some of the things that we are proudest of developing here in the company are scanning methods that allow imaging at 15 to 20 frames per second in the coronary arteries and catheters that are probably the smallest available," said LightLab CTO Joseph Schmitt.
LightLab has also placed about 15 gastrointestinal OCT imagers at selected sites through Asahi Pentax, which is running the clinical end of that program, Schmitt said. While the GI imaging engine is basically the same as in the cardiovascular systems, the catheters and accessories are quite different for the two systems, he said. A LightLab-Lantis OCT prototype targeted for the highly cost-sensitive dental market, has begun the evaluation process at University of California–San Francisco, Schmitt said.—Hassaun A. Jones-Bey
Dual approach measures health of engineered tissue
In the pursuit of alternatives to organ and tissue transplants, researchers are developing so-called tissue-engineered medical products (TEMPs), which are a combination of a three-dimensional organic scaffold and tissue grown upon it. In just one example, Cook Biotech (Bloomington, IN) manufactures TEMPs fabricated from the submucosal layer of porcine tissues, upon which tissue is grown for the repair of wounds, burns, and hernias. Knowing in detail how well the tissue functions upon the scaffold, however—and therefore its readiness for use—is not an easy task.
Scientists at the National Institute of Standards and Technology (NIST; Gaithersburg, MD) have combined two pre-existing imaging technologies—optical coherence microscopy (OCM) and confocal fluorescence microscopy (CFM)—whose properties complement each other for this application. Whereas OCM provides cellular images with very good rejection of light scattered out of the focal plane, and has a large (greater than 100-dB) dynamic range, CFM has relatively poor background rejection but provides crucial information on the viability of cells from fluorescence measurements.
The light source for the system's OCM segment is based on a semiconductor optical amplifier and supplies 10 mW of optical power centered on 1310 nm and with a 70-nm bandwidth. The OCM's interferometer is fiberoptic and constructed from single-mode polarization-maintaining fiber; its sensor is a coherence-domain reflectometer. Output light from the OCM segment is collimated by a free-space lens. Supplied with 488-nm light from an argon-ion laser, the CFM segment contains a photomultiplier tube for sensing. The outputs from the two systems are combined with a dichroic beamsplitter and sent through a 1.3-numerical-aperture oil-immersion objective. The systems' foci are well aligned laterally but, due to their differing wavelengths, were initially offset axially by 5 µm—a displacement later corrected optically to within 1 µm, says NIST scientist Joy Dunkers.
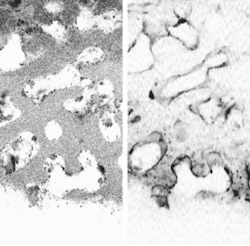
The OCM and CFM components can be viewed either together or separately (see figure). When cross-sectional planes of scaffolding and tissue are captured at progressively larger depths using the combined OCM and CFM information, a movie can be constructed that depicts a journey through the TEMP. The reachable imaging depth is proportional to the sample porosity and inversely proportional to the refractive-index mismatch between the scaffold and the immersion medium, explains Dunkers. For example, a partially hydrated collagen scaffold allows imaging to about 500 µm."The goal of using both OCM and CFM is to extract not only the structure of the scaffold and the location of cells using OCM, but to use the CFM as a barometer to tell us how 'happy' the cells are in the scaffold," says Dunkers. "We have fixed the cells and stained only for the presence of nuclei. We could also stain for the presence of certain proteins or extracellular matrix that is expressed to demonstrate the viability of cells. We could use a membrane stain to show us the shape of the cells. And, yes, in certain applications where we would just like to see cell migration, we may not need to stain at all and hence no CFM component is needed." Ultimately, the researchers want to characterize live cells in situ.—John Wallace

John Wallace | Senior Technical Editor (1998-2022)
John Wallace was with Laser Focus World for nearly 25 years, retiring in late June 2022. He obtained a bachelor's degree in mechanical engineering and physics at Rutgers University and a master's in optical engineering at the University of Rochester. Before becoming an editor, John worked as an engineer at RCA, Exxon, Eastman Kodak, and GCA Corporation.
Hassaun A. Jones-Bey | Senior Editor and Freelance Writer
Hassaun A. Jones-Bey was a senior editor and then freelance writer for Laser Focus World.