Nanosphere (Northbrook, IL) has developed a workstation that uses silver-coated gold nanoparticles to allow the simultaneous detection of multiple genetic targets with a single test. Comprising two core instruments—the Verigene Reader and the Verigene Processor—and a single-use test cartridge, the Verigene system is a workstation for nucleic acid (DNA or RNA) and protein diagnostics.
Both the Verigene system and its first test—which assesses an individual’s ability to metabolize the blood-thinning medication warfarin (sold under the brand name Coumadin) received FDA clearance last year. (Warfarin is the most prescribed oral anticoagulant in North America and Europe, and the second most common cause of emergency room-visits for adverse drug events.) Since then, Nanosphere has had other tests approved, and the company has gone public.
How it works
A medical technician uses a pipette to insert a small amount of tissue sample into the cartridge, uses the Verigene Reader to correlate the sample to the test cartridge, and then loads the cartridge into the Verigene Processor, which images the slide and analyzes the results.
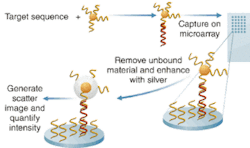
According to Bill Cork, vice president of research and development and the company’s chief technical officer, the entire optics system, all reagents, and the gold nanoparticles are contained within the disposable cartridge. In use, the assay captures an analyte on a glass substrate, the target binds with it, and then the gold nanoparticle complex binds with the target. The analyte is an oligonucleotide in the case of a nucleic acid test, and an antibody in the case of the protein test, typically, he says.
The glass substrate acts as a waveguide. “We actually use the slide as a sort of fiber-optic cable,” he explains. Light is provided by LEDs through a waveguide that illuminates the edge of the slide. “We put imaging controls on four corners so we make sure we’ve got even illumination” across the plate, says Cork. Depending on the critical entry angle of the light, a certain amount will “leak out” of the glass surface and form an evanescent wave. “What’s important is that it is the source of illumination,” says Cork.
The gold nanospheres captured in the assay are 13 nm in diameter and don’t scatter much light individually, so the company developed a process to coat the nanospheres with silver and thus amplify the light. “Typically silver won’t bind itself to gold,” Cork says, “but with our process the silver will autonucleate to the gold. So we end up with particles that are upward of one-half to one micron, and highly reflective.” Light coming off the waveguide scatters off the silver-coated particle and creates “a one-micron sphere of light that we can detect optically. We use a simple CCD camera and we see bright stars where gold nanoparticles are.”
“Our arrays are about 10 × 25 spots,” which are typically 100 to 150 µm in diameter. Target capture analytes collect in each of those spots which, when hit with the evanescent wave, “look like a bright white spot in field of darkness.” Verigene is “at least 100 times more sensitive” than other techniques, says Cork. And reportedly the technology is also 100,000 times more selective, with far fewer false positives and false negatives. Furthermore, the technology promises far faster results than traditional systems—and at significantly less cost; Nanosphere says hospitals will be able to afford the technology and keep sample processing in-house instead of sending out to labs as they now do.

Barbara Gefvert | Editor-in-Chief, BioOptics World (2008-2020)
Barbara G. Gefvert has been a science and technology editor and writer since 1987, and served as editor in chief on multiple publications, including Sensors magazine for nearly a decade.