OPTOELECTRONIC APPLICATIONS: IN VIVO IMAGING: Microendoscopy takes a practical turn
Discussions about how to motivate the medical community to embrace new technologies often focus on form and function—user-friendliness, compactness, ergonomics, speed, cost, and so on. For biomedical optics, these discussions further require demonstrating that a laser-based device can do something more-conventional approaches cannot, or at least do it better (such as laser refractive surgery, skin rejuvenation, hair removal, and optical coherence tomography). But with the advent of collaborative R&D projects that put engineers, physicists, and biologists in the same room with pathologists, urologists, neurologists, oncologists, and other medical experts, product and application development increasingly involves looking at how a surgeon or physician functions in the operating room or at the patient bedside, and how an optical tool or technique can enhance the delivery and outcome of a procedure—that is, the “people” side of the equation.
This trend has had an important effect on the development of microendoscopic tools for in vivo imaging. At Cornell University (Ithaca, NY), for example, multiphoton-microscopy pioneer Watt Webb and colleagues in the Department of Applied Physics have been collaborating with biochemists, urologists, neurologists, endoscopists, and surgeons at Weill Medical College in New York City to develop endoscopic multiphoton imaging devices that can provide information noninvasively about a potential cancer—including its size, depth, and location—reducing or even eliminating the need for multiple physical biopsies and enabling more accurate presurgical planning. According to Webb, multiphoton microendoscopy in the 750 nm range (most often utilizing a Ti:sapphire laser) shows promise as a noninvasive visualization tool for assessing and monitoring cancer and other diseases in the brain, bladder, esophagus, and upper and lower GI tracts.
“We decided to start with urology, particularly inside the bladder,” Webb says. “There are all kinds of bladder cancer, and 80% to 90% of them are superficial cancers. Traditionally these are discovered by taking biopsies and waiting several days for the results. But taking a bladder biopsy is very traumatic, and sometimes several biopsies are needed to determine exactly where and how big the cancer is. Each biopsy requires hospitalization and anesthesia, and if the results are positive you have to wait at least two weeks before you can surgically approach the cancer because the patient has to recover from the biopsy surgeries. We are looking at using multiphoton microendoscopy to nondestructively image the bladder surface and find the cancer so it can be treated right away.”
Commercial success
OptiScan (Melbourne, Australia) has also taken an applications-oriented approach to developing the market for what it terms “endomicroscopy,” although the company uses confocal rather than multiphoton techniques. OptiScan’s first product, the research grade Benchtop F900e, was released in 1995. Designed to convert a conventional microscope into a 3-D confocal system, it has been in use for several years in many leading research organizations and hospitals. The company subsequently released the Rigid Probe (in 1998) as an attachment to the Benchtop F900e to enable imaging of living tissue in vivo, and the Hand Held Probe for applications in dermatology, particularly the detection and monitoring of skin cancers and inflammatory skin conditions.
But OptiScan’s biggest successes to date have come through its licensing relationships with Pentax (Tokyo, Japan) and Carl Zeiss (Oberkochen, Germany). OptiScan has been working closely with Pentax since 2002 and Zeiss since mid-2007, as well as key researchers and clinicians in Germany and the United States, to identify specific medical applications and to customize its technology for both flexible (Pentax) and rigid (Zeiss) endoscopes. The OptiScan products utilize a 480 nm blue solid-state laser, a full-screen CCD detection system, and nine different lenses in the probe. The Pentax endoscope is a 38 French 12.8 mm scope, with a 0.5 × 0.5 mm field of view, 1000× magnification, and 250 µm penetration depth. It works in conjunction with a contrast agent in the blue spectrum to create the tissue fluorescence to be detected.
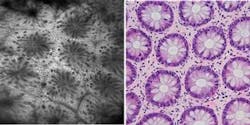
Since its release in March 2006, studies have shown that the OptiScan/Pentax confocal endomicroscope (the Pentax ISC-1000) enables in vivo microscopic imaging of the upper and lower GI (gastrointestinal) tract in histological detail at the time of patient examination without the collection of biopsies (see Fig. 1). Preliminary data from blind clinical studies suggests that the use of this device can increase the diagnostic yield for disease detection in conditions such as ulcerative colitis and Barrett’s esophagus, in which diagnosis using white-light endoscopy is problematic.1 Other potential applications for this technology under investigation include the trachea, duodenum, small bowel, and colon. OptiScan is also conducting preclinical trials in gene therapy, molecular imaging, blood-flow imaging, and vascular-response monitoring.
“These systems are not like magic wands that are going to say ‘is this cancer or not’—they are visualization tools,” says Peter Delaney, director of technology at OptiScan. “The big problem with histopathology today is sampling. If you don’t have the right bit of tissue, you aren’t going to do any good. And unless you know the basis for taking out a tissue biopsy, you are inherently going to be low on specificity. The importance of endomicroscopy is that it is a histological tool that can help endoscopists make intraoperative decisions about whether to take a biopsy, and better identify where biopsies should be taken. This, in turn, can reduce the number of biopsies needed.”
Next year’s models
A number of microendoscopy R&D efforts are also focused on neuroimaging. Mark Schnitzer and colleagues in the Departments of Biological Sciences and Applied Physics at Stanford University (Stanford, CA) have developed several one- and two-photon fluorescence microendoscopy systems that enable minimally invasive in vivo imaging of cells within deep brain tissues with micron-scale resolution (see www.laserfocusworld.com/articles/241169). Conventional two-photon microscopy is only able to penetrate about half a millimeter below the tissue surface; to get at deeper structures, the Stanford team inserts tiny fiber-optic probes into the brains of laboratory animals to produce, for example, detailed images of minute cerebral blood vessels located more than 1 mm below the surface.
“We seek to provide the resolution of a fluorescence microscope in a minimally invasive instrument based on micro-optics and fiber optics. This will allow us to image cells with micron-scale resolution in places where microscopes cannot go,” says Schnitzer, who is also a faculty member of the neuroscience, biophysics, and molecular imaging programs in the Stanford School of Medicine.
Schnitzer’s group first published on the use of microendoscopy to study the brain in 2003; in that paper, they demonstrated that what many had thought might be critical obstacles in the delivery of ultrashort pulses to deep tissues—specifically, chromatic dispersion and self-phase modulation—could be readily avoided. The key was the use of gradient-index (GRIN) lenses, which are also often easier and cheaper to produce than conventional stand-alone microlenses.2
“A key advantage of GRIN lenses is that they can be economically made in large numbers and at high numerical apertures without grinding them into shape,” Schnitzer says. “The face of a GRIN lens is typically cut flat. That means you can prepare long glass rods, control the degree of ion doping in the rods, and then dice up the rods into lenses.”
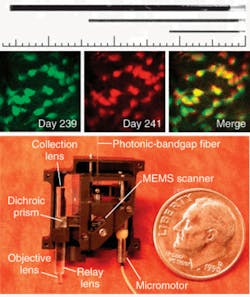
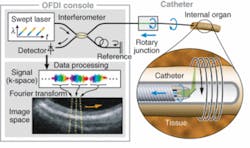
More recently, Schnitzer’s group demonstrated the use of MEMS scanners to further reduce the size of two-photon microendoscopy devices (see Fig. 2). Ultrashort pulses from a Ti:sapphire laser are delivered by a hollow-core photonic-bandgap fiber that virtually eliminates ultrashort pulse distortion. The illumination deflects off the MEMS mirror, which scans the light in a raster pattern. The light then enters an optical assembly consisting of a dichroic microprism and four microlenses. Fluorescence is generated in the sample at the focal spot of the objective lens, passes back undeflected through the microprism, and is focused by a collection lens into a multimode optical fiber that routes to a photodetector. Schnitzer’s group is now applying this technology to applications in basic neurobiology and clinical settings.3“We believe that by using OFDI we will one day be able to visualize plaques and treat them based on what we see,” Tearney says. “We are the only academic institution developing OFDI for intercoronary use, and the first to do this in humans. We think this is going to revolutionize cardiovascular medicine.”
REFERENCES
1. M. Canto et al., Gastrointestinal Endoscopy 65, AB346 (2006).
2. J. C. Jung and M. J. Schnitzer, Optics Lett. 28, 902 (2003).
3. W. Piyawattanametha et al., Optics Lett. 31, 2018 (2006).
4. D. Yelin et al., Nature 443, 265 (2006).
5. W. H. Yun et al., Nature Medicine 12, 1429 (2006).
Kathy Kincade | Contributing Editor
Kathy Kincade is the founding editor of BioOptics World and a veteran reporter on optical technologies for biomedicine. She also served as the editor-in-chief of DrBicuspid.com, a web portal for dental professionals.