BIOPROCESS MONITORING: Photonics takes a new look at bioprocesses
MARK SELKER AND BARB PALDUS
More than a third of all drugs under development are biotechnology based. Because biological processes involve synthesis of large and complex molecules such as monoclonal antibodies or recombinant proteins in live cells, sophisticated manufacturing methods are required to optimize the yield of production runs. The yield of bioprocesses depends on the viability and growth rates of the cell lines, while reproducibility depends on the controlled variation of the bioreactor process conditions that determine the cellular metabolic state and productivity.
Real-time monitoring used in commercial bioreactors includes dissolved oxygen (DO), pH, temperature, and pressure. Cellular parameters, such as cell mass and viability, are obtained using off-line procedures with bioreactor sampling occurring every 4 to 24 hours. But as precision and speed requirements on critical bioprocess sensors become more stringent, traditional electrochemical and off-line methods will no longer suffice. Therefore, optical methods are being developed (see Fig. 1).
Physical process parameters
Measurement of process variables such as temperature and pressure rely on well-established techniques. For these parameters, sensor precision, accuracy, response time, and stability are adequate for bioprocess control.
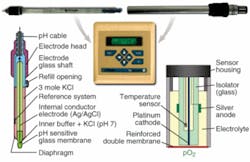
A pH electrode determines the hydrogen ion (H+) concentration potentiometrically—the underlying principle resembles a battery—while dissolved oxygen or polarographic sensors are based on a Clark cell (see Fig. 2).Both pH and DO measurements can be improved. The precision of electrochemical sensors is adequate but their response time is slow (less than 60 s), and their readings drift over time as components age or active membranes become coated with cells. These sensors also require calibration before each use and regular off-line reference standardization. Furthermore, the lithium-doped glass used for the construction of pH probes is often brittle and can break inside the bioreactor during a growth run; similarly, DO-sensor glass electrodes often crack during autoclaving. Because of these disadvantages, current electrochemical cells do not address the needs of modern bioprocessing.
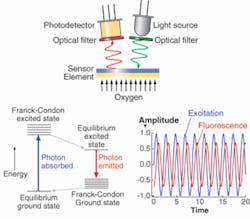
Phase fluorimetry can provide an alternative. These sensors utilize the fact that the fluorescence lifetime of an indicator dye can be quenched by an analyte of interest. The dye or dyes are entrapped in an analyte-permeable porous matrix. Depending on the analyte, the sensing schemes will use one dye or ratio of the signals from two different dyes.1 In the simplest case, the dye lifetime is monitored as it is quenched by the target analyte. The quenching process used to sense DO is described (to first order for dye in a solvent) by the Stern-Volmer equations:
- I/I 0 = 1 + K svP O2 (1)
- τ/τ 0 =1 + K svP O2 (2)
- K sv = kτ 0 (3)
where Ksv is the Stern Volmer constant, k is the diffusion-dependent bimolecular quenching constant, I is fluorescence intensity, τ is optical transition lifetime, and pO2 is oxygen partial pressure.2 The subscript 0 represents the unquenched state (no oxygen present).
As can be seen from equations 1 and 2, the quenching process affects both the fluorescence lifetime and the intensity. Either can be used to measure the degree of quenching and therefore the concentration of the analyte of interest. In practice, fluorescence lifetime is the more practical indicator. The use of phase fluorimetry eliminates problems from drift in source intensity, surface irregularity, and other issues. The transfer function of the dye response looks very similar to a low-pass filter; when the pump light is modulated, the output fluorescence signal is lower in amplitude and is phase delayed, such that:
- Tan φ = ωτ(p O2) (4)
While phase fluorimetry is conceptually straightforward, it is not entirely free of issues. The electronics can be challenging and the dyes degrade with exposure to the analyte, the excitation light, and the ambient light. The degradation is generally due to singlet-triplet crossing and the formation of photo-stable ground states of other species. Methods to reduce the rate of photodegradation include minimizing the exposure to ambient light, addition of singlet oxygen inhibiting components, and minimizing the excitation intensity.3 These limitations can be managed and are offset by the advantages of a simple, rugged sensing element. Other advantages include faster response times (less than 45 s), the ability to precalibrate, the ability to presterilize in situ (disposable bioreactors, for example), and cost per use. Hence, optical DO and pH sensors are gaining popularity for bioprocessing applications.
Cell parameters
In a bioprocess, the viable cell density (VCD) is defined as the number of live cells per unit volume, while total cell density (TCD) is the total number of cells per unit volume, including dead cells and detritus. In cell culture, VCD is a critical parameter. In the early stages, when TCD is low and nutrients are abundant, most of the cell population is viable, so TCD approximates VCD. As the culture reaches completion, large numbers of cells begin to die. Knowing the VCD is important for optimizing harvest time and maximizing yield.
Measurements of TCD and VCD are made using off-line laboratory cell counting methods. For TCD the number of cells in a known volume is physically counted, while VCD is measured using the Trypan blue-dye exclusion method. Here, cells with compromised membranes (that is, nonviable cells) will absorb the dye and become stained. The VCD is then computed as the difference between TCD and stained cell density.
Traditionally, TCD was measured by counting cells manually with a light microscope and a clicker-counter, while for VCD a light microscope and a hemacytometer was used. Today, instruments obtain a video image of the sample before and after automated mixing with Trypan blue dye and use automated counting algorithms to determine TCD and VCD. Nevertheless, off-line sampling and sample dilution are still required, so accuracy and precision remain limited by human error. In addition, these off-line methods incur time delays between sampling and results, so they cannot be used for real-time bioprocess control. In general, it is also labor intensive and risky to repeatedly pull samples from a bioreactor.
Optical cell-density probes are enjoying increased popularity because they provide real-time, in situ process data. The optical cell-density probe, like many traditional electrochemical probes is inserted into the bioreactor and autoclaved with the bioreactor before use. It resides inside the bioreactor during the growth run and gives a real-time continuous reading.
Most optical cell-density probes have their genesis in wastewater applications where turbidity probes characterize the quality of the water and its treatment. Unfortunately, the term turbidity is so loosely defined that these devices are generally not immediately applicable to bioprocessing. Optical cell-density probes often utilize Mie scattering to account for cell density. The optical loss across a known distance gives a measure of the suspended-particle density. It is also possible to use back- and side-scattered light to get similar information. In the simplest case, light is sent across a gap and the optical loss is measured. If a bioprocess is stable and repeatable, the real-time optical-loss measurement can be correlated to off-line cell-density measurements. This means that through simple nonlinear mapping, the transmitter can display the actual cell-density units for future growth runs.
We should note here that the industry-standard use of absorption units (AU) for these probes instead of optical density (OD) is somewhat ambiguous and highlights the confusion that exists within the bioprocessing industry. It is our contention that an optical scattering measurement should isolate a single variable, namely scattering loss. The most efficacious measurement is one that strives to measure only the scattering loss due to the cells. However, the current off-line method, often called “OD 600” or “OD 550,” in which the number gives the spectrophotometer wavelength in nanometers, underscores this issue. As many biological entities absorb at 550 nm (near the solar emission peak), one can easily envisage a scenario in which the growth media absorption changes in this spectral region, while the cell density remains the same; this would clearly produce an erroneous change in the perceived cell density. Additional complications arise when different spectrometers are used, as the AU level at which the forward-scattering saturation occurs can change from instrument to instrument. The AU reading will then depend on where it is measured. Given these issues, we believe that physical cell count or dry cell weight is the best “gold standard” reference method for calibrating an optical cell-density probe.
Another optical probe of interest is a cell-viability probe. Chance was the first to note that in situ culture fluorescence can provide a window into cell metabolism.4 Specifically, both NADH and NADPH (nicotinamide adenine dinucleotides) fluoresce near 460 nm with the NADH fluorescence stronger than NADPH. Apparently, the corresponding oxidized forms do not fluoresce. It has been suggested that the ratio of these reduced nucleotides to their oxidized forms can be used to determine cellular “health.”5 Several attempts have been made to construct a functioning probe, but have yet to yield a commercial success.
Optical techniques allow bioengineers to perform real-time, noninvasive interrogation of their bioprocess state and thus more effectively and cost-efficiently characterize each growth run. Future bioprocessing will undoubtedly use optical methods to either upgrade traditional sensors or enable new ones, enabling dramatic improvements in process yield.
REFERENCES
1. C. Huber et al., Anal. Chem. 73, 2097, (2001).
2. J.R. Lakowicz, Principles of Fluorescence Spectroscopy, 2nd Edition, Springer Science (2004).
3. L.F. Capitan-Vallvey et al., Analytica Chimica Acta 583(1) 166 (2007).
4. D.E.F. Harrison and B. Chance, Appl. Microbiol. 19, 446 (1970).
5. K. Andersen and K. von Meyenburg, J. Bio. Chem. 252, 4151 (1977).
MARK SELKER is CTO and vice president of sensors and Barb Paldus is CEO at Finesse Solutions, 3350 Scott Blvd, Bldg 1, Santa Clara, CA 95054; e-mail: [email protected]; www.finesse.com.