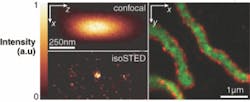
The imaging resolution of far-field fluorescence microscopy techniques is limited by the point-spread function (PSF) of the focal spot, which quantifies the blur of the object point in an image. Today’s gold standard, the confocal microscope, has a cigar-shaped, diffraction-limited PSF greater than 200 nm in diameter in the focal plane (x, y), and greater than 500 nm along the optical axis (z), or roughly comparable to the fluorescence wavelengths used for imaging (400 to 700 nm). By using stimulated emission depletion (STED), however, a subdiffraction-size PSF can be achieved. Extending the STED method further to create an isotropic (uniform in all directions) spherical nanosize focal spot, researchers at the Max Planck Institute for Biophysical Chemistry (Göttingen, Germany) and the German Cancer Research Center (Heidelberg, Germany) can perform three-dimensional (3-D) cell imaging with a resolution of 40 to 45 nm using this new “isoSTED” technique.1
In STED microscopy, the fluorescence-excitation light is overlapped with another light distribution that features a local intensity zero, such as an annular distribution (a cylinder along the optic axis having a doughnut-shaped cross section). Excited fluorophores in the sample to be imaged are quenched by the overlapping light distribution except at the zero, essentially confining fluorophore excitation to the now very narrow PSF zero region. While other techniques such as molecular switching can achieve the same effect, they require very bright fluorophore signals and are limited to thin sample layers.
An unfortunate limitation of the standard STED technique is that the use of a doughnut still renders a very asymmetric focal spot because the doughnut squeezes the spot just in the focal plane, but not along the z-axis. The result is a spot extending as a thin rod along the z-axis that, while providing subdiffraction resolution in the focal plane, does not yield any improvement along the z direction.
Hollow sphere of light
To concurrently gain the same resolution in the z direction, the research team set out to create a spherical subdiffraction-size spot. Using two opposing lenses that coherently add the wavefronts in the focal region (a so-called “4Pi microscope”), the researchers have now created a nearly spherical focal spot that delivers an isotropic spatial resolution of 40 to 45 nm. The secret to creating the spherical PSF—which appears as a 3-D doughnut that encloses the imaging area—is to add the intensity of two different lasers to create a hollow sphere of light that replaces the doughnut cylinder used so far. The isoSTED concept was implemented in a commercially available confocal laser scanning microscope (Leica TCS 4Pi from Leica Microsystems in Mannheim, Germany) providing the rapid beam-scanning and detection units.
Using the isoSTED microscopy setup, the distribution of mitochondrial proteins in mammalian cells was investigated. The 200-to-400-nm-diameter spherical- or tubular-shaped mitochondria organelles contain different types of proteins that can be labeled with different fluorophores. Cross-sectional analysis of the mitochondria shows clustering of certain proteins at the boundary of the mitochondria, while other proteins collect in the center of the organelles (see figure). The isoSTED technique was able to image these mitochondria in 3-D at a resolution of roughly 50 nm. To the knowledge of the researchers, this is the first time that light microscopy has recorded two separate proteins inside an organelle within a whole cell at nanoscale 3-D resolution.
“A unique strength of the isoSTED method is that the nanosize focal spot can be arbitrarily targeted to any coordinate inside the cell at high speed, thus providing rapid scans from the cellular interior with unprecedented far-field 3-D resolution,” says Stefan Hell, Max Planck professor. “Despite the roughly tenfold improved spatial resolution along the z axis over confocal microscopy, the spot can be further substantially reduced in size,” adds Alexander Egner, senior scientist. “In the future, one will be able to combine isoSTED with very rapid image recording.”
REFERENCE
1. R. Schmidt et al., Nature Methods 5, 539 (May 18, 2008).
About the Author

Gail Overton
Senior Editor (2004-2020)
Gail has more than 30 years of engineering, marketing, product management, and editorial experience in the photonics and optical communications industry. Before joining the staff at Laser Focus World in 2004, she held many product management and product marketing roles in the fiber-optics industry, most notably at Hughes (El Segundo, CA), GTE Labs (Waltham, MA), Corning (Corning, NY), Photon Kinetics (Beaverton, OR), and Newport Corporation (Irvine, CA). During her marketing career, Gail published articles in WDM Solutions and Sensors magazine and traveled internationally to conduct product and sales training. Gail received her BS degree in physics, with an emphasis in optics, from San Diego State University in San Diego, CA in May 1986.