ALBERT A. RUTH and JOHANNES ORPHAL
Fourier-transform spectroscopy (FTS) is a well-established spectroscopic method (based on Michelson interferometry) that was developed about 50 years ago to improve and complement the performance of grating or prism spectrometers.1 The technique has been used in fundamental metrology for the measurement of the speed of light, for the definition of the meter, for the definition of wavelength standards, and for the establishment of accurate reference spectra and energy levels of key species in astrophysics and atmospheric remote sensing. Due to their rapidity, simplicity, and robustness, FTS instruments are also used in many industrial applications, in particular in the near- and mid-IR regions.
More recently, highly sensitive spectroscopic approaches have been developed that use optically stable cavities with high-reflectivity mirrors as sample cells that detect very weak absorptions (down to 10-10 cm-1 and below).2 There are several variations using pulsed or continuous-wave lasers, known as cavity ring-down spectroscopy (CRDS) or cavity-enhanced absorption spectroscopy (CEAS; also called integrated-cavity output spectroscopy), which have been used extensively in the last decade to measure ultra-weak absorptions in the laboratory and in the field.3-5
White-light sources
In 2003 it was shown that incoherent white-light sources can be used in conjunction with optical cavities, leading to so-called incoherent broadband cavity-enhanced absorption spectroscopy (IBB-CEAS).6 The latter method is comparable to the well-known intracavity laser absorption spectroscopy (ICLAS), except that the absorbing medium is not contained within a laser cavity but in an external cavity that is excited with a broadband light source.7 Because IBB-CEAS setups are experimentally simple and robust, the method is particularly suited for field sensing. It is particularly relevant for applications requiring broadband coverage and high time resolution, such as absorption measurements in liquids and on surfaces.
In the past, the combination of optical cavities with Fourier-transform spectrometers has been mainly based on ICLAS. Although the first experimental implementation of FTS in combination with laser-based CRDS was reported more than ten years ago, and more recently a cavity-enhanced phase-shift setup was demonstrated using an incoherent light source, the advantages of using FTS in combination with broadband cavity-enhanced techniques had not been fully exploited before now.8, 9
However, it is straightforward to combine FTS and IBB-CEAS to form a very simple and robust experimental setup with several advantages. Using a standard light source and a small optical cavity (with a length of 90 cm and a diameter of 4 cm, for example), absorption spectra can be obtained that are equivalent (in terms of spectral resolution and signal-to-noise ratio) to spectra recorded with multipass-absorption cells that have a volume approximately 1000 times larger.
This volume advantage makes Fourier-transform cavity-enhanced absorption spectroscopy (FT-CEAS) very interesting for a variety of applications in which the sample volume is intrinsically small (for example, flames, plasmas, and chemical-flow reactors) or when samples cannot be used in large volumes (such as explosives, corrosive substances, and rare and expensive isotopically substituted molecules). All the benefits of FTS compared to dispersive spectrometers (such as throughput, spectral resolution and coverage, and spectral calibration) are maintained.
FTS with broadband light
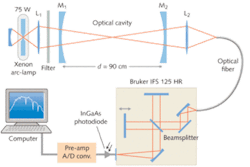
In the experiment described here, the Fourier-transform spectrometer was a Bruker IFS 125 HR (see Fig. 1) with a dielectrically coated calcium fluoride beamsplitter and a maximum spectral resolution of about 0.001 cm-1. The detector was an indium gallium arsenide (InGaAs) photodiode at room temperature with high sensitivity in the near-IR. The broadband light source was a stabilized “super-quiet” 75 W xenon-arc lamp. The optical cavity was formed by two dielectric mirrors with a radius of curvature of 2 m and a specified reflectivity of 99.7%.
The mirrors were attached to a stainless-steel tube of 4 cm diameter and were separated by 90 cm; the resulting sample volume was about 1100 cm3. An external-cavity diode laser facilitated aligning the optically stable cavity. The mirror reflectivities were accurately determined by introducing a known quantity of a gaseous absorber into the cavity. To limit the spectral bandwidth to the region of high mirror reflectivity, optical bandwidth filters centered at approximately 1600 nm with a full-width at half-maximum (FWHM) of about 100 nm were placed into the light beam in front of the cavity. The range of the filters where the transmission drops to 1% was of about 300 nm.
The photons exiting the cavity were focused into a multimode optical fiber (0.6 mm core diameter, 5 m length, numerical aperture 0.22) that coupled the light into the source compartment of the Bruker FTS. The optics were mounted on a fully adjustable optical holder and care was taken to homogeneously illuminate the entrance aperture of the FTS.
Broad coverage, high sensitivity
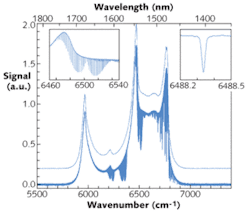
High-resolution spectra of the evacuated optical cavity and of the cavity filled with 26.7 mbar of pure carbon dioxide (CO2) were taken in the region between 5800 and 7000 cm-1 (about 1350 to 1800 nm; see Fig. 2). The spectral resolution of the CO2 spectrum is about 0.02 cm-1 (the spectra shown were recorded at a resolution of 0.015 cm-1–the Doppler-width of CO2 in the near-infrared region is about 0.013 cm-1); the spectrum was obtained from the average of 200 interferometer scans corresponding to an acquisition time of about 90 minutes. The spectrum of the empty cavity (recorded at a reduced resolution of 4 cm-1) is shifted upward by 0.2 divisions for clarity in the figure.The data demonstrate the combined advantages of broadband coverage, high spectral resolution, and high sensitivity when using FTS together with IBB-CEAS. While the sensitivity is modest in comparison with CRDS experiments (a value of 7.5 × 10-6 cm-1 Hz-1/2 was determined for the noise-equivalent absorption from the 1σ rms value of the noise at 6665 cm–1, region without an absorption feature), the advantage of this approach lies in the very broad coverage at high resolution.10 Note that even with the small sample cell in this setup, one can achieve an effective absorption length of about 370 m.
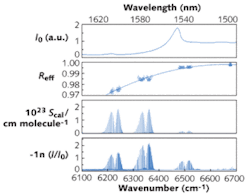
Mirror reflectivity is an important experimental parameter in cavity-enhanced spectroscopy. It is possible to determine the effective mirror reflectivity from high-resolution absorption measurements (see Fig. 3), if a calibration gas with well-known absorption-line strengths is used. With CO2 as the calibration gas and the HITRAN database (www.cfa.harvard.edu/hitran) as reference for the line strengths, the effective reflectivity can be determined over a very broad spectral region (more than 100 nm) in a single measurement.The spectra demonstrate the potential of combining FTS with IBB-CEAS, especially when small sample volumes are required. The measurements highlight the usefulness of this approach for spectroscopic investigations of isotopic or dangerous samples, electric or plasma discharges, flames, or chemical sources like flow tubes in a steady state. The broadband high-resolution spectra of molecular lines obtained with a calibration gas provide an improved way to calibrate effective mirror reflectivities (or absolute absorption cross-sections and line strengths) without the need for additional measurements using an empty cavity. This method is less suited, however, for kinetic studies because of the relatively low time resolution (a typical spectrum is recorded in 90 minutes).
The use of alternative light sources such as LEDs or supercontinuum sources can increase the signal-to-noise ratio by at least one order of magnitude. This will certainly in the near future boost the use of incoherent broadband cavity-enhanced absorption spectroscopy and Fourier-transform spectrometers for a wide variety of scientific and analytical applications.
ACKNOWLEDGMENTS
The authors would like to acknowledge support by the Irish Environmental Protection Agency grant 2008-FS-EH-2-S5 and the European Marie Curie Transfer of Knowledge programme grant MTKD-CT-2004-014406.
REFERENCES
- S.P. Davis et al., Fourier Transform Spectrometry, Academic Press (2001).
- A. Paldus and A.A. Kachanov, Can. J. Phys. 83, p. 975, 2005.
- A. O’Keefe and D.A.G. Deacon, Rev. Sci. Instrum. 59, p. 2544 (1988).
- A. O’Keefe, Chem. Phys. Lett. 293, p. 331 (1998).
- R. Engeln et al., Rev. Sci. Instr. 69, p. 3763 (1998).
- S.E. Fiedler et al., Chem. Phys. Lett. 371, p. 284 (2003).
- K. Strong et al., Appl. Opt. 36, p. 8533 (1997).
- R. Engeln and G. Meijer, Rev. Sci. Instr. 67, p. 2708 (1996).
- E. Hamers et al., Chem. Phys. Lett. 365, p. 237 (2002).
- J. Orphal and A.A. Ruth, Opt. Express, 16, p. 19232 (2008).
Albert A. Ruth is a senior lecturer in experimental physics in the Department of Physics, National University of Ireland, University College Cork, Cork, Ireland; e-mail: [email protected]. Johannes Orphal is a professor of physics at the Laboratoire Interuniversitaire des Systè mes Atmosphériques (LISA), Université de Paris-Est, CNRS UMR 7583, Créteil, France.