OPTOFLUIDICS: 'Spatially modulated emission' advances point-of-care diagnostics
PETER KIESEL, JOERG MARTINI, MARKUS BECK, MALTE HUCK, MARSHALL BERN, and NOBLE JOHNSON
Today the majority of biological and biomedical tests are performed at major, centralized laboratories because compact, robust, and inexpensive instruments for point-of-care (POC) testing are simply not available. Yet there is a need for POC testing: The strategic landscape is undergoing a truly disruptive transformation. Driving this need are reduced costs, timely test results, lower mortality rates, and reduced morbidity.
Flow cytometers are indispensable tools in centralized laboratories, but their cost, complexity, and size preclude their use for POC diagnostics and environmental or industrial monitoring. Flow cytometry is the process of measuring chemical and/or physical characteristics of biological cells as they flow through an instrument in a fluid stream.1,2 Virtually all modern commercial instruments rely on optical interaction with the bioparticles for characterization through fluorescence, scattering, or absorption processes. And all use the same basic optical configuration-namely, intense illumination of the bioparticle as it speeds (at typically 6 m/s) through a highly localized spot, which generally involves a complex arrangement of optics (lenses, mirrors, apertures, and filters). Over the past decade many interesting concepts have been developed to simplify and miniaturize on-the-flow analyte characterization by utilizing microfluidic channels that integrate fluidic handling (pumping, mixing, flow focusing) and miniaturized optics.3,4 Despite this effort, a compact and low-cost instrument is not commercially available. To overcome this deficiency and meet the needs for POC testing, the Palo Alto Research Center (PARC) has introduced a radical redesign of the optical detection system for flow cytometry.
Spatially modulated fluorescence detection
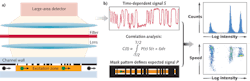
Called "spatially modulated emission," our technique delivers high signal-to-noise discrimination without precision optics to enable a flow cytometer that combines high performance with robustness, compactness, low cost, and ease of use. The enabling technique generates a time-dependent signal as a continuously fluorescing bioparticle traverses a predefined pattern for optical transmission. Correlating the detected signal with the known pattern achieves high discrimination of the particle signal from background noise.
In conventional flow cytometry, the size of the excitation area is restricted approximately to the size of the particle. Our method uses a much larger excitation area to increase the total flux of fluorescent light that originates from a particle. Despite the large excitation area, the mask pattern enables high spatial resolution in the micron range. This allows for independently detecting and characterizing particles with a separation (in flow direction) that can approach the dimension of individual particles. In addition, the concept is intrinsically tolerant to background fluorescence originating from other fluorescent components in solution, fluorescing components of the chamber, and contaminants on the surface.
To apply the spatially modulated fluorescence emission technique to particles moving through a fluid channel, a spatially patterned mask modulates the intensity of the fluorescent light incident on the photodetector over a large excitation area (see Fig. 1).5 The time dependence of the signal is defined by the spatial structure of the stripes of the mask and the speed of the particle. The recorded signal is analyzed by correlation techniques, and the intensity and time when the particle traverses the detection zone are accurately calculated. With state-of-the-art real-time correlation techniques, characterization is possible for particle speeds up to a few meters per second.
A compact prototype
Using off-the-shelf components, PARC has assembled and tested a handheld flow cytometer based on the spatial modulation technique (see Fig. 2). The largest component is a low-cost 532 nm laser module with the beam directed through the polished end facet of the fluidic chip onto the approximately 1.2 × 0.1 mm detection area. The fluidic chip is integral to the detection unit (see Fig. 3). The assembly includes inexpensive collimator optics, a filter for excitation light, and a compact silicon photodiode with integrated transimpedance amplifier and collection optics. The patterned mask is embedded in the fluidic chip; therefore, alignment between fluidic chip and detector unit is unnecessary. In the current version the only required alignment is directing the laser beam to the detection area, which is relatively uncritical because of its large size. The whole unit is battery powered, with two 9 V and four 1.5 V batteries sufficient for more than 10 hr of continuous operation.
By taking advantage of volume discounts on components and assuming volume production, a unit manufacturing cost target for a handheld POC device of a few hundred dollars seems realistic. The anticipated price target is extremely favorable in comparison to that of any commercially available or publicly announced device. For fluidic handling and data collection we currently use a commercial syringe pump and a desktop computer. As a next step, the fluidic handling will be simplified by using an inexpensive spring-loaded syringe and data evaluation will be performed by a notebook computer. A future possibility to implement data evaluation could be a field-programmable gate array (FPGA) or the use of a smart phone. The fluidic chips used for the prototype are in-house fabricated and assembled from two acrylic slides with a laser-machined adhesive film. Future fluidic chips are anticipated to be disposable and fabricated by high-volume, low-cost fabrication techniques like injection molding or hot embossing.
Demonstrating performance
The standard method to demonstrate the performance of a flow cytometer uses small-diameter beads impregnated with a specific fluorophore in a known concentration.6 A frequently used fluorophore for this purpose is Phycoerythrin (PE) dye, and the unit of fluorescent intensity is "molecules of equivalent PE" or MEPE, which defines the brightness of the micro-beads.
The detection range of our compact prototype was evaluated with 2 μm diameter rainbow calibration beads (RCP 20-5 from Spherotech in Lake Forest, IL). A detection limit of better than 300 MEPE was demonstrated by measuring a total of 10,420 beads in 10 μl of solution, which took less than 10 min (see Fig. 4).
The absolute calibration in MEPE was obtained by cross-calibration with commercially available calibration beads with specified PE intensity (BD Quantibrite from BD Biosciences; San Jose, CA).
The recorded total particle counts were in excellent agreement with the expected values except for the one peak at the low-intensity end of the range. This deviation from the expected value identifies the detection limit. However, the demonstrated sensitivity of our prototype is already sufficient to meet the needs of a wide range of bioparticle detection applications, including human blood analysis.
CD4 in whole blood
To demonstrate the utility of the technology, measurements were performed of absolute CD4+ counts in human blood, which are required for screening, initiation of treatment, and monitoring of HIV-infected patients. For benchmarking we performed a direct one-to-one comparison of measurements on the same blood samples with our prototype and a commercial instrument, the FACSCount from BD Biosciences. The whole-blood samples were tagged with a standard CD4 reagent (PE-CD4, PE/CY5-CD3 and known number of fluorescent micro-beads). With this protocol, no lysis (cell disruption) of the red blood cells or washing step to remove unbound dyes is necessary.
Sample preparation was performed at both PARC and BD Biosciences for comparison with the recommended sample preparation protocol. A histogram of detected particles as a function of fluorescent intensity shows two peaks that identify the calibration beads and the CD4+ T-lymphocytes (see Fig. 5). The signature for CD4 monocytes is also visible at the low-intensity end of the histogram. For the measurement, approximately 20 μl of analyte solution that contains approximately 2 μl of whole blood (dilution 1:10) were analyzed in less than 10 min. Based on the known concentration of calibration particles, the absolute concentration of CD4+ T-lymphocytes in the whole blood was determined. The resulting concentration of 670 ±15 CD4/μl is in excellent agreement with the 682 CD4/μl determined with the FACsCount, which demonstrates the utility of spatially modulated fluorescence emission as a practical solution for handheld point-of-care diagnostics.
ACKNOWLEDGMENTS
The authors are pleased to acknowledge support and helpful discussions with Robert Hoffman and Nga Bui from BD Biosciences for the CD4 benchmarking experiments. The work was partially supported by a grant from the National Institutes of Health (NIH).
REFERENCES
1. H.M. Shapiro, Practical Flow Cytometry, Wiley & Sons (2005).
2. M.A. Van Dilla et al., Science, 163, 1213 (1969).
3. D.A. Ateya et al., Analytical and Bioanalytical Chem., 391, 1485 (2008).
4. J. Godin et al., J. Biophoton., 1, 355 (2008).
5. P. Kiesel et al., Appl. Phys. Lett., 94, 041107 (2009).
6. R.A. Hoffman, Springer Series on Fluorescence, 6, Springer-Verlag Berlin Heidelberg, 307-342 (2008).
Peter Kiesel is principal scientist, Joerg Martini is scientist, Markus Beck is post doctorate, Malte Huck is research assistant, Marshall Bern is principal scientist, and Noble Johnson is manager of the optoelectronics group at the Palo Alto Research Center (PARC), 3333 Coyote Hill Rd., Palo Alto, CA 94304; e-mail: [email protected]; www.parc.com.