ORGANIC LASERS: Living fluorescent-protein-doped cell lases
It’s alive, and it lases! Single living cells genetically engineered to produce a fluorescent protein have operated as tiny living lasers when placed in a resonant cavity with a high quality factor (Q) and optically pumped with nanosecond nanojoule pulses.1 The cells not only survived but also made better lasers than solutions containing the protein.
Laser action has been demonstrated in a wide range of materials in the past half-century, including organic materials. But the experiments performed by Malte Gather and Seok Hyun Yun of the Wellman Center for Photomedicine at Massachusetts General Hospital (Boston, MA) mark the first time a living cell has served as a laser medium. The two physicists are also affiliated with Harvard Medical School (Boston, MA).
“Part of the motivation of this project was basic scientific curiosity,” says Gather, the lead author. “We wondered whether there was a fundamental reason why laser light, as far as we know, does not occur in nature, or if we could find a way to achieve lasing in biological substances or living organisms.”
Their starting point was the green fluorescent protein, discovered decades ago by Osamu Shimomura in a bioluminescent jellyfish.2 Reactions with calcium ions in the jellyfish excite the protein to fluoresce in the blue-green, but in the lab blue or ultraviolet light produces fluorescence peaking at 509 nm. Fluorescence had long been known from organic dyes, but Shimomura’s discovery was the first fluorescent protein.
Fluorescent protein rather than dye
Although many organic dyes fluoresced brightly across the visible spectrum, their toxicity posed problems for biomedical researchers using them for fluorescent imaging of living cells. The fluorescent protein avoided that problem, and genetic engineering made it possible to produce it in other types of cells. In the 1990s, a technique was devised to splice DNA coding for the green fluorescent protein into genes coding for another protein, so the genes fused the two proteins together. This allowed researchers to trace the native protein by monitoring the fluorescence of the attached protein. Roger Tsien at the University of California–San Diego then developed enhanced versions of the protein and shared the 2008 Nobel Prize in chemistry with two other researchers for its development.
A 2002 study reported two-photon-excited laser emission from the green-fluorescent protein in solution.3 Gather and Yun first tried single-photon pumping of a red fluorescent protein with a Q-switched 532 nm laser. However, they switched to the green fluorescent protein after they dug out an old tunable optical parametric oscillator (OPO) that could pump the green protein at 465 nm. When they filled the 7 mm cavity between a pair of mirrors highly reflective in the green, they observed fluorescence spread across 37 nm. The linewidth narrowed to 12 nm when the 5 ns pulses from the OPO exceeded the protein’s 14 nJ laser threshold.
To make living lasers, they infused plasmids carrying genes for the green fluorescent protein into a line of cells derived from embryonic human kidney cells. They then placed single cells containing the green fluorescent protein in a medium inside a high-Q cavity formed by a pair of distributed Bragg reflectors separated by 20 µm. The cells were about 15 µm in diameter but did not touch the cavity surfaces.
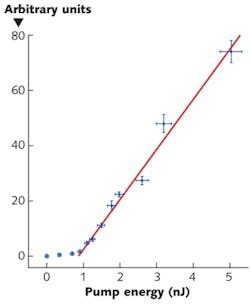
Focusing the OPO pulses onto the cell through a microscope objective, they observed laser threshold near 1 nJ, more than an order of magnitude lower than in solution (see figure). Gather and Yun attribute the low threshold to the refractive index of the cell being higher than the surrounding medium, so they focused the light in a plane-parallel mirror resonator that was “otherwise only marginally stable,” they write in Nature Photonics. Just above threshold, the width of the 516-nm-wavelength laser line was less than the 0.04 nm resolution of their spectrometer, but multiple lines appeared at higher pump powers, and they observed complex mode patterns, which changed little between pulses.
Remarkably, the cells survive their lasing experience. “We found no indication that cell viability is affected by lasing,” write Gather and Yun. Killing cells took pump powers orders of magnitude higher than what was used in the laser experiment. Bleaching required hundreds of pulses at a 10 Hz repetition rate.
The experiment attracted wide attention in the popular press for its novelty. But the ability to induce lasing in living cells without harming them opens new possibilities in bioimaging. The emitted mode patterns depend strongly on refractive index, so they could reveal 3D structures inside cells. The bright, directional fluorescence could increase the speed of flow cytometry measurements. They could even have therapeutic applications. “The ability to generate laser light from a biocompatible structure placed inside a patient could be useful for photodynamic therapies,” says Yun. In the long term, Gather hopes, living lasers might provide an interface between electronics and living organisms.
REFERENCES
1. M. Gather and S.H. Yun, Nature Photon., June 12, 2012; doi:10.1038/NPHOTON.2011.99.
2. O. Shimomura et al., J. Cell. Compar. Physiol., 59, 223–239 (1962).
3. D.J. Pikas et al., J. Phys. Chem. B, 106, 4831–4837 (2002).

Jeff Hecht | Contributing Editor
Jeff Hecht is a regular contributing editor to Laser Focus World and has been covering the laser industry for 35 years. A prolific book author, Jeff's published works include “Understanding Fiber Optics,” “Understanding Lasers,” “The Laser Guidebook,” and “Beam Weapons: The Next Arms Race.” He also has written books on the histories of lasers and fiber optics, including “City of Light: The Story of Fiber Optics,” and “Beam: The Race to Make the Laser.” Find out more at jeffhecht.com.