PHOTONICS APPLIED: BIOPHOTONICS: Gradient field microscopy allows label-free disease diagnosis
Imaging unstained live cells and thin slices of tissues (biopsies) is extremely challenging because these structures are transparent and, as a result, the images lack contrast. Over the past four centuries, much of the development in light microscopy has been driven by this challenge of obtaining high-contrast images of translucent structures.1
Contrast-generating microscopy methods fall into two classes: extrinsic and intrinsic, depending on whether the approach requires exogenous contrast agents or not, respectively. In the first category we find methods that involve stains—such as those used in standard clinical pathology—and fluorescence dyes, used extensively in cell biology. Intrinsic methods include phase contrast microscopy and differential interference contrast (DIC) microscopy.2 These approaches exploit the particular interaction between light and tissue rather than attaching absorbers or emitters to the structure of interest. Therefore, intrinsic contrast methods have the advantage of studying the cells and tissues in their unperturbed conditions.
Quantitative phase imaging (QPI) is emerging as an intrinsic contrast method that measures how much the light is delayed through the specimen at each point in the field of view.3 This optical path length or phase information relates to both the refractive index and thickness of the specimen and therefore enables new biological studies—especially of cell structure and dynamics.4-6 Recently, our group at the University of Illinois at Urbana-Champaign discovered that QPI holds valuable potential for clinical pathology; that is, the optical path-length map associated with the biopsy can be used for cancer diagnosis using spatial light interference microscopy, or SLIM.7
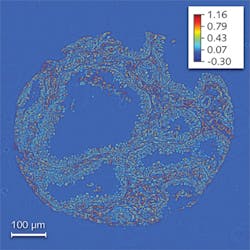
The SLIM method combines Zernike’s phase contrast microscopy and Gabor’s holography to render quantitative phase images, and is sensitive to path-length changes of 0.3 nm spatially and 0.03 nm temporally.8 We obtained images of prostate biopsy cores that were diagnosed by the pathologist as high-grade prostatic intraepithelial neoplasia (HGPIN) using a 40X/0.65 numerical aperture (NA) objective (see Fig. 1).
Gradient field microscopy
Remarkably, we found that the spatial fluctuations of the path length and not its average values hold the true diagnosis power for this microscopy method. In other words, while the average phase shifts in the tumor and normal tissue have similar values, the statistics of the spatial fluctuations (such as the variance) are completely different. Specifically, we found that the architecture of the prostate tumor is more disordered and characterized by higher variance as compared with the normal tissue, which is smoother. These results may form the basis for a new, label-free, quantitative approach to cancer diagnosis.
From an optical point of view, the fact that only relative changes in the phase shift and not absolute values are relevant to tissue diagnosis is quite significant. It suggests that new, simpler methods can be developed that do not necessarily quantify phase shifts but instead measure some spatial differential of the phase map. One such method is gradient field microscopy (GFM), a technique recently developed at our Quantitative Light Imaging Laboratory (http://light.ece.illinois.edu/).9 The GFM method provides high-contrast images of transparent specimens, including cells and tissues. In GFM, the spatial change of the optical path length is measured by taking the first- or second-order derivatives of the phase information that is carried by the light.
Currently, GFM is built as an add-on module to a commercial brightfield microscope. The aperture stop is closed down for the highest possible spatial coherence of the illumination. The system consists of a series of two lenses forming a 4f system to provide access to the Fourier transform of the image field. At this Fourier plane, spatial amplitude modulation is provided through a spatial light modulator. Depending on the modulation mask given at this plane, the type (and order) of the phase derivative obtained at the image plane changes. There are three different modes of GFM that we have implemented to obtain different phase derivatives: a first-order derivative along one direction (one gradient component), the amplitude of the first-order derivative (gradient intensity), and a second-order derivative (Laplacian).
GFM advantages
The GFM setup brings many advantageous features such as fast acquisition speed and stability along with the flexibility of using the different modes of operation. Since GFM is a single-shot technique and does not require any image processing after acquisition, the acquisition speed of this system is as fast as the camera allows. Furthermore, the spatial modulation can be changed electronically, without moving mechanical parts of the system. This contrasts with the conventional DIC microscopy technique in which the physical movement of a birefringent prism is required. The absence of the prism in the imaging system also allows GFM to image through birefringent materials such as plastic-bottom dishes, which is generally difficult using DIC (see Fig. 2). Cells exhibiting low contrast under common DIC microscopy are rendered visible via GFM.Our current work focuses on identifying “optical markers” for diagnosis and automating the process. We anticipate that based on such advanced imaging principles, “smart microscopes” could be developed in the near future that are capable of yielding knowledge rather than images. That is, we believe it is possible to have an imaging system that performs real-time analysis and diagnosis without ever saving the images. The combination of advanced optical imaging and parallel image processing shows the promise of revolutionizing standard pathology, allowing for faster, label-free diagnosis—affordable at the global scale.
ACKNOWLEDGMENTS
This research was supported by the National Science Foundation (grants CBET 08-46660 CAREER, CBET-1040462 MRI) and National Cancer Institute (R21 CA147967-01). For more information, go to http://light.ece.illinois.edu/. The authors are grateful to Krishna Tangella and Andre Balla for help with pathology expertise.
REFERENCES
1. Editorial, “Milestones in light microscopy,” Nature Cell Biol., 11, 1165 (2009).
2. M. Pluta, Advanced light microscopy, Polish Scientific Publishers, Warsaw, Poland (1988).
3. G. Popescu, Quantitative phase imaging of cells and tissues, McGraw Hill, New York, NY (2011).
4. Y.K. Park et al., Proc. Natl. Acad. Sci., 107, 15, 6731–6736 (2010).
5. H.F. Ding et al., Phys. Rev. Lett., 101, 23, 238102 (2008).
6. M. Mir et al., Proc. Natl. Acad. Sci. 108, 32, 13124–13129 (2011).
7. Z. Wang et al., J. Biomed. Opt., 16, 11, 116017 (2011).
8. Z. Wang et al., Opt. Exp., 19, 2, 1016–1026 (2011).
9. T. Kim, S. Sridharan, and G. Popescu, Opt. Exp., 20, 6, 6737–6745 (2012).
10. J.I. Epstein and G.J. Netto, Biopsy Interpretation of the Prostate, Lippincott Williams & Wilkins, Philadelphia, PA (2007).
11. J.I. Epstein and M. Herawi, J. Urol., 175, 820–834 (2006).

Dr. Gabriel Popescu, Ph.D. | Assistant Professor in the Department of Electrical and Computer Engineering
Professor Gabriel Popescu (1973-2022) received his B.S. and M.S. in Physics from University of Bucharest, in 1995 and 1996, respectively. He obtained his M.S. in Optics in 1999 and Ph.D. in Optics in 2002 from the School of Optics/ CREOL (now the College of Optics and Photonics), University of Central Florida. Dr. Popescu continued his training with the G. R. Harrison Spectroscopy Laboratory at MIT, working as a postdoctoral associate. He joined the University of Illinois at Urbana-Champaign (UIUC) in August 2007 as an Assistant Professor in the Department of Electrical and Computer Engineering and held a full faculty appointment with the Beckman Institute for Advanced Science and Technology. He was also an affiliate faculty in the Bioengineering Department. Dr. Popescu received the 2009 NSF CAREER Award, was the 2012 Innovation Discovery Finalist selected by the Office of Technology Management Office, UIUC, and was elected as the 2012-2013 Fellow of the Center for Advanced Studies at UIUC. Dr. Popescu was an Associate Editor of Optics Express and Biomedical Optics Express, and Editorial Board Member for Journal of Biomedical Optics.
Dr. Popescu passed away June 16, 2022.
Taewoo Kim | Graduate Student, Beckman Institute for Advanced Science and Technology at the University of Illinois at Urbana-Champaign
Taewoo Kim is a graduate student in the Department of Electrical and Computer Engineering & Bioengineering and the Beckman Institute for Advanced Science and Technology at the University of Illinois at Urbana-Champaign (Urbana, IL).
Shamira Sridharan | Graduate Student, Beckman Institute for Advanced Science and Technology at the University of Illinois at Urbana-Champaign
Shamira Sridharan is a graduate student in the Department of Electrical and Computer Engineering & Bioengineering and the Beckman Institute for Advanced Science and Technology at the University of Illinois at Urbana-Champaign (Urbana, IL).