COMPUTATIONAL IMAGING: Lens-free on-chip microscope is field-portable
ALON GREENBAUM, UZAIR SIKORA, and AYDOGAN OZCAN
Brightfield microscopy is widely used in various fields, including biomedicine. Nevertheless, a fundamental limitation of optical microscopes is that they have a limited field of view (FOV), which makes it labor intensive, tedious, and relatively expensive to detect rare microscopic features of interest (for example, abnormal cells or signatures of parasites). Another limitation is their relatively bulky structure, which makes the technique less suitable for field use.
Digital in-line holography
To address these limitations, lens-free holographic on-chip imaging techniques can provide high-resolution images over large sample areas using compact, lightweight, and cost-effective designs.1-4 In one example of a lens-free holographic microscope, the underlying operation principle is based on partially coherent digital in-line holography, where light-emitting diodes (LEDs) are used for illumination (see Fig. 1). Butt-coupled to multimode optical fibers, each LED illuminates the specimen with an effective aperture size of about 0.1 mm. This illumination configuration ensures that the light impinging on the specimen, which is positioned very close to the image sensor, is sufficiently coherent that the scattered object field can interfere with the background (that is, unscattered) light.
The resulting interference pattern encodes the phase information of the object in the form of an in-line hologram sampled using, for example, a CMOS sensor array. The same hologram-recording geometry, under unit fringe magnification, can also handle the relatively large bandwidth of the source without sacrificing spatial resolution. On the other hand, the pixel size at the sensor array presents a challenge for improving spatial resolution to submicron range.
To mitigate this sampling limitation, we use an array of LEDs, which are individually turned on and off to shift the lens-free in-line holograms of the objects at the sensor plane. Based on pixel super-resolution techniques, we can synthesize an in-line hologram that has effectively much smaller pixel size, yielding sub-micron spatial resolution across an FOV of, for example, 30 mm2, which is more than 100 times larger than the FOV of a typical brightfield microscope with a comparable resolution.1,2,5 As a proof of concept, such lens-free holographic on-chip microscopes based on partially coherent in-line holography were used, for example, for imaging of malaria parasites, performing cytometry on a chip, and high-throughput detection of waterborne parasites.1,6,7
Multiheight phase recovery algorithm
Relatively recently, the same platform has been modified to better handle dense and confluent samples, which present challenges for lens-free on-chip imaging in general due to its transmission geometry. To address the image distortion that occurs for dense samples, a multiheight phase recovery algorithm was implemented in partially coherent in-line holography.8-10
This algorithm requires a few intensity measurements acquired at different sample-to-sensor distances. Each measured in-line hologram is pixel-super-resolved independently, after which phase recovery is iteratively achieved by propagating (using the angular-spectrum approach) back and forth among these different super-resolved planes (see Fig. 1a). This iterative process neither assumes prior information about the sample dimensions nor imposes a spatial mask for affecting the convergence of the algorithm. Instead, it reinforces the super-resolved field amplitude of each hologram plane while converging on the unknown object phase.
In our field-portable design, a Z-shift stage was implemented to obtain different intensity measurements at different heights (see Figs. 1b and 1c). This stage is based on a nut-and-screw principle, where the CMOS image sensor is positioned over the moving nut while the screw is stationary. As the Z-shift knob is manually turned, the distance of the image sensor to the sample is decreased or increased. This design is cost effective and has a precision on the order of 10–15 μm. On the other hand, the exact Z shifts do not need to be known apriori since they can be digitally estimated by applying an autofocus algorithm.7
Pap smears imaged
To validate the performance of this field-portable microscope, liquid-based Papanicolaou tests (Pap smears) were imaged. The Pap smear/test is considered to be one of the gold-standard tests for cervical cancer screening, which is the second most common cancer among women worldwide. Cervical cancer leads to about 0.3 million deaths each year around the world, especially affecting developing countries where prescreening programs are not enacted.
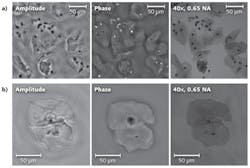
Figure 2a shows the backpropagated lens-free images that were obtained with our field-portable microscope, using five intensity measurements acquired at different heights. The sample is a 2D confluent Pap smear (the SurePath preparation, produced by BD of Franklin Lakes, NJ), and only a small portion (30 mm2) of the reconstructed FOV is shown. For comparison, a 40X (0.65 numerical aperture) objective lens image of the same FOV is also provided. Note that the inner morphology of the cells shows an enhanced contrast in the amplitude image, while the boundaries of the cells are better resolved in the phase image. This might facilitate the calculation of the nuclear-to-cytoplasm ratio (NC ratio) of these cells, where a high NC ratio might indicate that a specific cell is abnormal or precancerous.To further validate the performance of the field-portable microscope, a different type of Pap test (the ThinPrep liquid preparation, by Hologic of Bedford, MA) was also imaged. The backpropagated amplitude and phase images and the corresponding 40X microscope objective image of this test are shown in Figure 2b. These results demonstrate that multiheight phase recovery is able to reconstruct samples with complex structure, without the need for spatial masking or filtering, which reveals the promising potential of this microscopy platform for pathology needs in resource-limited settings.
REFERENCES
1. W. Bishara et al., Lab Chip, 11, 1276 (2011).
2. W. Bishara et al., Opt. Expr., 18, 11181 (2010).
3. D. Brady, Optical Imaging and Spectroscopy, John Wiley & Sons, NJ (2009).
4. W. Xu et al., Proc. Nat. Acad. Sci., 98, 11301 (2001).
5. R.C. Hardie et al., Opt. Eng., 37, 247 (1998).
6. C. Oh et al., Opt. Expr., 18, 4717 (2010).
7. O. Mudanyali et al., Lab Chip, 10, 2419 (2010).
8. L.J. Allen et al., Opt. Commun., 199, 65 (2001).
9. A. Greenbaum et al., Opt. Expr., 20, 3129 (2012).
10. A. Greenbaum et al., Lab Chip, 12, 1242 (2012).
Alon Greenbaum, Uzair Sikora, and Aydogan Ozcan are at the electrical engineering department of the University of California-Los Angeles (UCLA). Aydogan Ozcan is also with UCLA's bioengineering department and its California NanoSystems Institute; e-mail: [email protected]; http://innovate.ee.ucla.edu and http://biogames.ee.ucla.edu/.