PHOTONICS APPLIED: BIOPHOTONICS: Breath analysis research approaches clinical practicality
MATTHEW BARRE and ERIC TAKEUCHI
Imagine a time when a trip to the doctor's office to evaluate your ailment doesn't involve a painful blood draw or the hassle of a urine sample, but only entails a simple request to blow into a tube. Or perhaps a trip to the doctor isn't required at all: What if an evaluation could be conducted remotely by blowing into an accessory on your cell phone? This scenario is becoming a reality as the burgeoning field of breath analysis provides new and, perhaps most important, noninvasive methods to detect abnormal function within the body. Many of the leading techniques involve optical and laser-based approaches that are providing new capabilities to bring breath analysis into a clinical setting.
The notion of detecting disease states in exhaled breath has existed for almost 2500 years. Hippocrates described specific correlations between breath aroma and disease in one of his many medical treatises; in 1784, Lavoisier and Laplace showed that respiration consumes oxygen and eliminates carbon dioxide; in 1897, Nebelthau showed that diabetics exhale acetone in their breath; and in 1971, Pauling used gas chromatography (GC) to detect over 250 compounds in exhaled human breath.
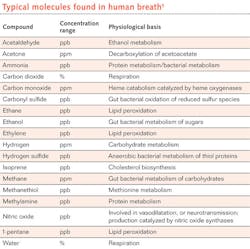
Since that time, advances in mass spectrometry (MS), combined GC-MS techniques, chemiluminescence sensors, electrochemical sensors, and infrared (IR) spectroscopy have allowed researchers to identify more than 5000 unique substances in exhaled breath. These substances include elemental gases such as nitric oxide and carbon monoxide as well as a wide variety of volatile organic compounds (see table).1
Biomarker identification
Current research in the area of breath analysis is heavily focused on the identification of specific biomarkers in exhaled breath that can be correlated to specific disease states in the body. In addition to the presence of breath acetone in diabetics as previously mentioned, many other breath biomarkers have been discovered, including nitric oxide for airway inflammation, hydrogen sulfide for periodontal disease, isoprene for cardiovascular disease, and numerous others.2
Many of these biomarkers are present in exhaled breath at extremely low concentrations, often at the part-per-million (ppm) and part-per-billion (ppb) levels. Drawing medically relevant conclusions from breath analysis requires accurate and repeatable measurements at these very low concentrations, and often with a complex mix of background gases and interferences.
Recent advances in analytical instrumentation have allowed researchers and clinicians to measure trace concentrations of these biomarkers, which have proven to be critical in the detection of normal and abnormal biological function.
While each of the established measurement techniques has its strengths and weaknesses, photonics-based breath analysis methods offer several unique advantages, including the ability to generate highly selective and sensitive results in real time. This real-time analysis provides rapid results and allows for the measurement of dynamic changes within a single breath. Real-time measurements can be especially critical in capturing the "end tidal breath," the air closest to the alveolar-capillary interface in the lung, which theoretically provides important information about metabolic processes in the blood.
Although most experts in breath analysis would claim that the field is still in its infancy, several companies have had commercial success. Most, if not all, of the products are focused on niche markets and use a highly customized sensor to assess a specific medical condition.
One example of a photonics-based product is the BreathTek Urea Breath Test (UBT) from the Medical Device Division of Otsuka America Pharmaceutical (Rockville, MD). The BreathTek UBT (www.breathtek.com) detects a biomarker in exhaled breath that indicates the presence of H. pylori bacteria in the gut, a leading cause of chronic gastritis and gastric ulcers. The system requires the patient to ingest a carbon-13 (13C)-labeled substrate and then measures the ratio of 13C to 12C in exhaled breath using nondispersive IR spectroscopy. Although this has proven to be an effective product for the detection of H. pylori and a validation of IR spectroscopy as a viable approach for breath analysis, additional development is required to realize a more broadly applicable diagnostic instrument.
Aerocrine (Solna, Sweden) has also seen commercial success with its NIOX line of nitric oxide monitoring devices. Nitric oxide (NO) production in vascular tissue has been shown to be intimately linked with inflammation. More specifically, the presence of excessive levels of cytokines, which are linked to the body's natural inflammatory response, stimulates the production of NO in tissue. The founders of Aerocrine contributed to research in the late 1990s that showed the levels of NO in exhaled breath could be used as a sensitive biomarker for inflammation in the airways.3 The company was founded in 1997 and now has a line of medical devices to accurately measure exhaled NO levels in the treatment of asthma and other allergic airway inflammation conditions.
The original Aerocrine devices were based on chemiluminescence but the newer devices use electrochemical sensors that have been proven to provide comparable results in a more compact, portable, and lower-cost package (see Fig. 1).4 Although these instruments have proven to be highly effective, the Aerocrine products are another example of the confinement of current breath analysis instruments to highly specialized niche markets.Mid-IR spectroscopy, colorimetric sensors, and optical frequency combs
One active area of breath analysis research is in the utilization of quantum cascade lasers (QCLs) as light sources for laser-based spectroscopy in the mid-infrared (mid-IR) "fingerprint" region. Broadly tunable external-cavity configurations can provide access to a wide range of spectral bands where many of the breath biomarkers have unique spectral responses. Boris Mizaikoff and his team at the University of Ulm in Germany recently published their research on the successful coupling of an external-cavity QCL with a miniaturized mid-IR hollow waveguide gas cell to quantitatively determine the 12CO2/13CO2 ratio within the exhaled breath of mice.5
Earlier research has shown that the isotope ratio of carbon-dioxide (CO2) isotopologues in exhaled breath can be used as an indication of glucose metabolism dysfunction. This condition is closely correlated with septic shock, which is the leading cause of mortality in intensive care units in the United States. Research such as this is driving the adoption of optical approaches to breath analysis, which could allow for real-time online monitoring of patients in a clinical setting.
Another optical approach to breath analysis that has seen recent progress is the colorimetric sensor array. These devices use an array of chemically responsive dyes that change color based on their chemical environment (see Fig. 2). After exhaled breath is drawn across the device, an optical image is collected and analyzed to determine the complex mixture of analytes that produced the composite color response. Peter Mazzone et al. recently published the results of their study using a colorimetric sensor array to identify exhaled breath biomarkers of lung cancer.6 The paper also reports recent advances in colorimetric sensor arrays, including robotic printing of reactive pigments to increase the surface area for reactions to occur as well as enhanced imaging technology to analyze the color responses.Diminishing barriers
Advances in sensor and instrumentation technology have helped to overcome some of the barriers that prevent broad adoption of breath analysis in clinical settings. Miniaturized, low-cost, portable instruments allow for the collection of breath data from large numbers of human subjects to support large, multi-institution clinical trials.8 In addition, emerging optical approaches allow for real-time analysis of breath samples, which reduces data-processing time and enables detailed analysis of the dynamics of a single breath.
Future progress will require standardization of breath collection and analysis methods, interaction with regulatory agencies, incorporation of personalized medicine approaches, and a continued multidisciplinary focus on sensor technology development and clinical trial validation.
Cristina Davis, PhD, a professor in the department of Mechanical and Aerospace Engineering at the University of California-Davis and host of the 2012 International Breath Analysis Meeting, emphasizes the importance of collaboration between industry and academia to make meaningful progress in the area of breath analysis. "Ultimately, larger-scale breath biomarker studies will need to be performed, and I envision that this will likely need to be at the public/private interface," says Davis. "I think academic research centers will need to form a national or international consortium with industry partners to conduct the large scale clinical trials that will be needed before breath testing routinely moves into clinical practice."
Dr. Raed Dweik, a leading researcher in breath analysis and the director of the Pulmonary Vascular Program at Cleveland Clinic (Cleveland, OH), states his opinion simply with a spin on a familiar quote: "Life is not measured by the number of breaths we take, but by the ones we analyze."
REFERENCES
1. T.H. Risby and F.K. Tittel, Optical Eng., 49, 11, 000000-1-000000-14 (November 2010).
2. F. Cikach and R. Dweik, Progress in Cardiovascular Diseases 55, 34–43 (2011).
3. R.A. Dweik et al., American J. Respiratory and Critical Care Medicine, 184, 602–615 (2011).
4. T. Hemmingsson et al., J. Clinical Monitoring and Computing, 18, 5-6, 379–387 (December 2004).
5. K. Wörle et al., "Breath Analysis with Broadly Tunable Quantum Cascade Lasers," Anal. Chem. (2013); doi:10.1021/ac3030703.
6. P. Mazzone et al., "Exhaled breath analysis with a colorimetric sensor array for the identification and characterization of lung cancer," J. thoracic oncology: official publ. of the Intl. Assoc. for the Study of Lung Cancer, 7, 137–142 (2011).
7. M. Thorpe et al., "Cavity-enhanced optical frequency comb spectroscopy: application to human breath analysis," Opt. Exp., 16, 2387–2397 (2008).
8. S. Solga and T. Risby, IEEE Sensors J., 10, 1, 7 (January 2010).
Matthew Barre is a business development manager and Eric Takeuchi is senior director of business development at Daylight Solutions, 15378 Avenue of Science, Suite 200, San Diego, CA 92128; e-mail: [email protected]; www.daylightsolutions.com.