OPTICAL COHERENCE TOMOGRAPHY: OCT advances into clinical applications
JUN ZHANG and ZHONGPING CHEN
Optical coherence tomography (OCT) has become a valuable noninvasive tool for many clinical applications. Among numerous technological advances in OCT, the recent development of new OCT light sources with long coherence length, broad scanning range, and ultrahigh scanning rate is particularly exciting. In addition, there have been significant advances in Doppler OCT, phase-sensitive OCT, and multimodal imaging.
Wavelength-swept sources
Microelectromechanical systems (MEMS) tunable vertical-cavity surface-emitting lasers (VCSELs) have recently attracted much attention due to their unprecedented coherence length. These MEMS-VCSELs were first demonstrated in the mid-1990s. In 2009, Praevium Research (Santa Barbara, CA), Thorlabs (Newton, NJ), Advanced Optical Microsystems (AOMicro; Mountain View, CA), and the Massachusetts Institute of Technology (MIT; Cambridge, MA) began an interdisciplinary effort to develop MEMS-VCSELs for swept-source OCT (SS-OCT) applications.
The advantages of MEMS-VCSELs for SS-OCT arise from the device structure, which is illustrated by a three-dimensional (3D) cutaway of a 1310 nm VCSEL (see Fig. 1).1 The short micron-scale cavity provides a free spectral range (FSR) greater than 150 nm, over which continuous mode-hop-free singlemode tuning can be obtained. This leads to dynamic coherence lengths exceeding 1 m. In addition, the small mirror mass translates to very high MEMS mirror mechanical resonance that enables axial scan rates exceeding 1 MHz. These devices have been demonstrated at both 1060 nm and at 1310 nm.
In addition to the MEMS-VCSEL, an all-electronic wavelength-tuning laser based on a Vernier-tuned distributed Bragg reflector (VT-DBR) structure was recently developed by Insight Photonic Solutions (Lafayette, CO). The source exhibits a single longitudinal mode providing a coherence length greater than 40 mm. Both 1550 nm and 1310 nm lasers of this type have the potential to be used as low-cost, high-performance swept sources for OCT.
Alternatively, a Fourier-domain modelocking (FDML) swept source that operates in a quasi-stationary regime based on an extralong laser cavity provides a broad sweep range (up to 200 nm) by combining two semiconductor optical amplifiers (SOAs) as the gain medium, with rapid scan rates up to 5.2 MHz through buffering the sweeps.2,3
Fourier-domain Doppler OCT
By combining Fourier-domain OCT (FD-OCT) with a phase-resolved method that basically uses a laser-induced Doppler frequency shift to obtain velocity flow information, Fourier-domain Doppler OCT enables high-speed and high-sensitivity Doppler imaging in 3D.
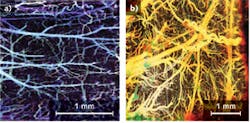
Thanks to its exceptionally high spatial resolution and velocity sensitivity, Fourier-domain Doppler OCT has been applied to numerous clinical applications. For example, Doppler OCT with an inter-frame scheme can image brain hemodynamics in the cerebral cortex.4 Doppler OCT images of an en face maximum intensity projection (MIP) microvasculature of mouse cerebral cortex with an intact skull can be compared to an en face MIP microvasculature of a rat cerebral cortex with a thinned skull (see Fig. 2).Another important application of Doppler OCT is the ability to create an optical angiogram to map the human retina and choroid microvascular network.5 Compared with conventional angiography methods such as fluorescein angiography and indocyanine green angiography that require the use of fluorescent dyes, the optical angiogram is superior with the advantage of label-free and 3D imaging.
Phase-sensitive FD-OCT
High-sensitivity phase measurement is an important technique for detection of nanometer- or sub-nanometer-scale displacements. Fourier-domain phase-sensitive OCT has excellent phase stability, high sensitivity, and high imaging speed for quantitative phase measurement. In this technique, the phase information of the complex depth-resolved profile is extracted by Fourier transformation of the spectral fringes generated by the optical path difference (OPD) between the reference and sample arms in the measurement setup. Since half a wavelength of the OPD results in a phase shift of 2π radians, an ultrahigh-accuracy measurement of the OPD with nanometer or sub-nanometer resolution can be achieved with high-sensitivity phase measurement of the fringes.
If the inherent barrier of 2π ambiguity is corrected by phase-unwrapping processes such as phase retrieval in the spectral domain, the phase-sensitive OCT system can measure a large range of displacements with picometer-range sensitivity.6 The phase-sensitive FD-OCT method has been recently applied to photothermal imaging and extraction of a photoacoustic signal. In the photothermal imaging experiment, integrated structural and molecular-targeted imaging for cancer markers (nanoshells) is demonstrated in breast tissue. And in the second experiment, the photoacoustic signal is extracted from the phase-time evolution of a SS-OCT spectral sweep, enabling all-optical noncontact OCT and photoacoustic imaging with close-to-shot-noise-limited phase-sensitive detection.
Multimodal OCT for intravascular imaging
Eighty percent of coronary events are caused by the rupture of a thin-cap fibroatheroma—a plaque with a large lipid or necrotic core covered by thin fibrous caps. The rupture of the vulnerable plaques can trigger thrombus formations that block blood flow within coronary arteries and subsequently result in acute coronary syndrome. Therefore, early detection of plaque lesions is very important in preventing the lethal consequences of atherosclerosis.
Diagnosis of the latent vulnerability of a plaque lesion relies on measurements of the structural, mechanical, and chemical composition of tissues. A miniature (0.69-mm diameter) integrated OCT/ultrasound (OCT/US) probing system was developed for assessment of plaques offering high resolution, which is essential for visualizing the microstructure of plaque, and large penetration depth, which is important for visualizing deep structures within the vessel wall.7 The integrated OCT/US probe provides both OCT and ultrasound imaging simultaneously with enhanced diagnostic accuracy, and significant reduction of both cost and the physician's time compared to using separate OCT and ultrasound probes.
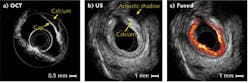
Optical coherence tomography and ultrasound provide complementary resolution and imaging depth. In a human coronary artery specimen with calcified plaque, OCT and ultrasound images combined are able to identify both the thickness of plaque cap and plaque extension (see Fig. 3). Although the OCT system can measure cap thickness, it could not image the entire vessel wall due to its limited penetration depth of about 1 mm. Ultrasound provides much larger penetration depths to image the full thickness of plaque. However, the relatively low resolution of ultrasound means that it cannot distinguish the calcium-tissue border adequately. OCT Medical Imaging Inc. (Irvine, CA) is currently developing integrated intravascular OCT/US technology for vascular clinical applications.Moreover, it is well known that an atherosclerotic plaque is stiffer than surrounding healthy tissues, meaning that the mechanical properties of the tissue can locate plaque sites. Optical coherence elastography (OCE)—which uses OCT data to map the mechanical properties of tissue—has superior, micrometer-scale resolution compared to other elastography techniques based on ultrasound and magnetic resonance imaging (MRI) modalities.8
A phase-resolved acoustic radiation force OCE (ARF-OCE) system using chirped ARF to excite a sample was recently shown to be able to delineate diseased tissue from normal tissue and quantitatively characterize the tissue's mechanical properties. For an atherosclerotic human coronary artery imaged using phase-resolved ARF-OCE, a strong vibration-phase contrast is achieved by applying a 500 Hz chirped acoustic radiation force upon the tissue, with phase shifts characterized by different colors (see Fig. 4). The region of less vibration represents less elastic, stiffer tissue—indicating atherosclerotic plaques.In addition, measurement of the biomolecular composition of atherosclerotic plaques provides important information including the presence of inflammation or the formation of a necrotic core toward diagnosis of the latent vulnerability of a plaque lesion. An OCT/fluorescence multimodal imaging system combining the high spatial resolution of OCT with the molecular sensitivity of fluorescence imaging can resolve both microstructure and biomolecular information at the same time.9 In an integrated SS-OCT and fluorescence intensity imaging system, a common fluorescence and OCT probe based on a double-cladding fiber combiner was developed to achieve real-time simultaneous OCT and superficial fluorescence intensity imaging.10Ex vivo imaging of rabbit arteries using the multimodal technique enabled complete characterization of vulnerable plaques.
REFERENCES
1. G. Overton, "MEMS-based VCSEL reaches record 150 nm tuning range," Laser Focus World, 48, 9, 10 (September 2012).
2. J. Zhang, G.J. Liu, and Z.P. Chen, "Ultra broad band Fourier domain mode locked swept source based on dual SOAs and WDM couplers," Proc. SPIE, 7554, 75541I–75541I-5 (2010).
3. W. Wieser et al., "Multi-Megahertz OCT: High quality 3D imaging at 20 million A-scans and 4.5 GVoxels per second," Opt. Expr., 18, 14, 14685–14704 (2010).
4. G. Liu et al., "Advances in Doppler OCT," Chinese Opt. Lett., 11, 011702–11712 (2013).
5. L. An and R.K. Wang, "In vivo volumetric imaging of vascular perfusion within human retina and choroids with optical micro-angiography," Opt. Expr., 16, 15, 11438–11452 (2008).
6. J. Zhang et al., "High-dynamic-range quantitative phase imaging with spectral domain phase microscopy," Opt. Lett., 34, 21, 3442–3444 (2009).
7. J. Yin et al., "Novel combined miniature optical coherence tomography ultrasound probe for in vivo intravascular imaging," J. Biomed. Opt., 060505 (2011).
8. W. Qi et al., "Phase-resolved acoustic radiation force optical coherence elastography," J. Biomed. Opt., 17, 110505 (2012).
9. H. Yoo et al., "Intra-arterial catheter for simultaneous microstructural and molecular imaging in vivo," NatureMedicine, 17, 12, 1680–1684 (2011).
10. S. Liang et al., "Intravascular atherosclerotic imaging with combined fluorescence and optical coherence tomography probe based on a double-clad fiber combiner," J. Biomed. Opt., 17, 7, 070501 (2012).
Jun Zhang is an assistant research professor and Zhongping Chen is a professor at the Beckman Laser Institute and the Department of Biomedical Engineering at the University of California-Irvine, Irvine, CA 92697. Chen is also a co-founder and chairman of OCT Medical Imaging Inc. E-mails: [email protected] and [email protected]; http://chen.bli.uci.edu.
![FIGURE 1. A solid model of the 1310 nm MEMS-VCSEL. The device combines an epitaxial half-VCSEL (including a GaAs/AlxOy DBR and InGaAs active region) with a dielectric suspended mirror structure. The structure is optically pumped through the top mirror. Wavelength tuning is realized via an integrated electrostatic actuator [1]. FIGURE 1. A solid model of the 1310 nm MEMS-VCSEL. The device combines an epitaxial half-VCSEL (including a GaAs/AlxOy DBR and InGaAs active region) with a dielectric suspended mirror structure. The structure is optically pumped through the top mirror. Wavelength tuning is realized via an integrated electrostatic actuator [1].](https://img.laserfocusworld.com/files/base/ebm/lfw/image/2016/01/1305lfw03f1.png?auto=format,compress&fit=max&q=45&w=250&width=250)