MICROSCOPY: Three-photon microscopy maps the brain
While two-photon fluorescence microscopy successfully enables depth imaging of cells and structures in scattering tissues, typical two-photon microscopy with a 775 nm laser source penetrates mouse brain tissue in vivo to depths of about 700 μm. By using longer wavelengths to reduce the attenuation of the excitation light by tissue, imaging depths can be extended. For example, balancing the tradeoff between mouse brain-tissue scattering and absorption results in an optimum calculated wavelength of 1700 nm to improve imaging depth.
Because hardly any fluorophores can be two photon-excited at 1700 nm and longer wavelengths alone cannot overcome tissue-scattering limitations, researchers at Cornell University (Ithaca, NY) are turning to the combination of longer-wavelength excitation and three-photon microscopy to reduce background scattering for improved deep-tissue imaging.1
Three photons
The fluorescence signal from three-photon excitation falls off as a function of 1/z4 (where z is the distance from the focal plane) compared to just 1/z2 for two-photon excitation, enabling three-photon excitation to dramatically reduce the out-of-focus background fluorescence in regions far from the focal plane. Furthermore, the amount of three-photon excitation increases when using a low-duty-cycle, high-pulse-energy excitation source with a low average power to prevent tissue heating. Combining these two attributes, the Cornell researchers developed a high-pulse-energy source at 1675 nm (close to the nominal 1700 nm for optimal brain-tissue imaging) via soliton-self-frequency shift (SSFS) in a photonic-crystal rod pumped by a high-energy, turnkey 1550 nm fiber laser.
The photonic-crystal rods’ large modefield area produces a high-energy soliton pulse for three-photon excitation: 67 nJ at a 1 MHz repetition rate—the highest soliton energy achieved, say the researchers, for SSFS in a solid-core fiber. The SSFS process both shifts the wavelength to 1700 nm (the optimal excitation wavelength for brain tissue) and compresses the pulse width by a factor of six to 65 fs (a peak power greater than 1 MW), a value more than adequate for deep-tissue three-photon excitation.
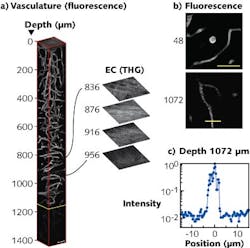
Using the source in a microscopy setup, mouse-brain vasculature labeled with a red fluorescent dye was imaged in vivo every 4 μm to a 1400 μm depth. The required power level at the surface of the mouse brain for three-photon microscopy was approximately 3 mW—comparable to traditional two-photon microscopy power levels. For the first 800 μm of imaging depth, power levels were adjusted to achieve the same signal-to-noise ratios as at the surface. But depths beyond 800 μm required the maximum source power of 22 mW at the sample surface and increased integration times.
Vascular imaging showed the blood vessels clearly at depths up to 840 μm below the surface of the brain (see figure). At this location (called the external capsule of the brain), a bright third-harmonic-generation (THG) signal indicates the beginning of the “white matter” region of the brain that is about 116 μm thick. At 956 μm below the surface of the brain, the hippocampus region appears. The researchers were able to image the blood vessels at depths to 1300 μm—nearly twice as deep as with state-of-the-art two-photon microscopy. Signal-to-background values at depths around 1000 μm are nearly an order of magnitude better compared to two-photon imaging at 1280 nm wavelengths, and calculations suggest these values could be even higher with a more optimum laser source.
In addition to vasculature, the researchers also successfully imaged red fluorescent protein (RFP)-labeled neurons deep inside intact mouse brain and are currently working on a tunable source with a range of 1650–1850 nm that will excite a larger number of fluorophores.
“For mapping the function of brain, optical imaging stands out because of its noninvasiveness and its capability to measure brain activity at high spatial and temporal resolution,” says Chris Xu, Cornell University associate professor. “In addition to improvements in terms of the laser source and the imaging optics, the development of robust functional probes at the orange or red spectral range offers the exciting possibility of mapping the activity of the brain beyond the superficial cortical layers. The brain contains many mysteries, and we hope this technology can be used to shed light on at least some of them.”
REFERENCE
1. N. G. Horton et al., Nat. Photon., 7, 205–209 (2013).
About the Author

Gail Overton
Senior Editor (2004-2020)
Gail has more than 30 years of engineering, marketing, product management, and editorial experience in the photonics and optical communications industry. Before joining the staff at Laser Focus World in 2004, she held many product management and product marketing roles in the fiber-optics industry, most notably at Hughes (El Segundo, CA), GTE Labs (Waltham, MA), Corning (Corning, NY), Photon Kinetics (Beaverton, OR), and Newport Corporation (Irvine, CA). During her marketing career, Gail published articles in WDM Solutions and Sensors magazine and traveled internationally to conduct product and sales training. Gail received her BS degree in physics, with an emphasis in optics, from San Diego State University in San Diego, CA in May 1986.