MICROSCOPY: 4Pi fluorescence microscope requires only a single objective lens
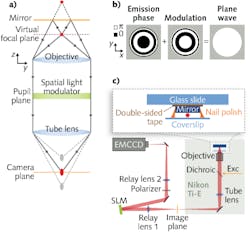
Conventional three-dimensional (3D) 4Pi microscopy for single-molecule and single-particle localization uses two objective lenses to improve the axial precision for a sample under test, which is usually about 2.5 times worse than the lateral precision.
In 4Pi microscopy, the emitted photons from a fluorescing particle or molecule are collected by two objectives and interfere at the camera plane, reducing the point-spread function (PSF) in the axial direction and improving axial resolution due to steeper intensity gradients. Unfortunately, each of the tens-of-centimeters-long interference arms requires subwavelength stability, which demands complex and expensive instrumentation. An alternative fluorescence-microscopy technique developed by researchers at the University of California, San Francisco (UCSF) uses a single objective to obtain 4Pi-comparable imaging resolution.1
Fluorescence emission, not source excitation
Unlike similar single-objective fluorescence self-interference and optical-trapping techniques developed to improve confocal and stimulated emission depletion (STED) microscopy that sandwich a sample between a single objective and a mirror and interfere the excitation light from the sample, the UCSF method instead interferes the fluorescence emission light from the sample. Two interference arms consist of the direct image of the sample and the mirror image of the emitter (see figure).
When the objective lens is focused on the mirror, the emitter image is in front of the camera plane and the mirror image is behind it. To interfere at the camera plane, both images must be in focus, which is achieved through placement of a spatial light modulator (SLM) in the pupil plane of the imaging path. By applying a SLM phase modulation that is the opposite of the calculated emission phase in the pupil plane, the modulation creates a plane wave that is focused at the image plane. Thus, the SLM effectively focuses the images at a virtual focal plane without any physical movement, whereas conventional methods use a mechanical positioner for focusing.
To verify the efficacy of the single-objective 4Pi setup, 100-nm-diameter fluorescent beads in aqueous solution excited with a 642 nm laser-diode source and positioned roughly 1 to 2 μm from the mirror on a coverslip were imaged with the system. The fluorescence signal was separated from the excitation light using a dichroic mirror and bandpass filter. To access the pupil plane, the image was relayed with lenses of 150 mm and 300 mm focal lengths onto a back-illuminated EMCCD camera. A 512 × 512 pixel SLM was positioned at the Fourier plane after the 150 mm relay lens.
Fluorescent-bead images from the single-objective interference setup show 4Pi-like PSFs with similar intensity oscillations as in a dual-objective 4Pi setup. “We were able to build this system on a commercial microscope frame, which makes it as easy to use as any other single objective setup, but with the benefits of two-objective interference,” says Joerg Schnitzbauer, graduate student at UCSF. “We hope that the simplicity of this solution will open new opportunities for everyday biological research that requires the best possible imaging resolution.”
REFERENCE
1. J. Schnitzbauer et al., Opt. Exp., 21, 17, 19701–19708 (2013).

Gail Overton | Senior Editor (2004-2020)
Gail has more than 30 years of engineering, marketing, product management, and editorial experience in the photonics and optical communications industry. Before joining the staff at Laser Focus World in 2004, she held many product management and product marketing roles in the fiber-optics industry, most notably at Hughes (El Segundo, CA), GTE Labs (Waltham, MA), Corning (Corning, NY), Photon Kinetics (Beaverton, OR), and Newport Corporation (Irvine, CA). During her marketing career, Gail published articles in WDM Solutions and Sensors magazine and traveled internationally to conduct product and sales training. Gail received her BS degree in physics, with an emphasis in optics, from San Diego State University in San Diego, CA in May 1986.