Spectroscopy: Ground-based VUV spectrometer supports satellite studies of planetary atmospheres
ADAM J. WISE
Isotope ratios skewed by natural fractionation processes allow scientists to reconstruct the evolution of planetary atmospheres. For example, Venus's deuterium-to-hydrogen (D/H) ratio is two orders of magnitude greater than that of Earth, possibly the result of a runaway greenhouse effect that boiled off Venus' oceans into space.
As deuterium—hydrogen's twice-heavier isotopic cousin—sluggishly escapes the planetary gravity well, D/H ratios hold a record of historical atmospheric fluxes. Especially valuable are measurements in the upper planetary atmosphere, where the fractionation process takes place.1 These D/H ratios can be measured remotely by quantitative photometry of atmospheric Lyman-α emission lines.
The discovery of anomalous D/H ratios in Venusian water vapor droplets by the Pioneer Venus mission (measured via mass spectrometry) stoked speculation about our other dry neighboring planet: Mars. The question of a once-blue Mars can be addressed via isotope measurements in addition to geological studies. Measurements of Martian D/H ratios were originally planned for the Japanese NOZOMI spacecraft (launched in 1998), but technical problems scuttled the mission, leaving the Martian D/H ratios an open question.2 Similar detection equipment was included in the Cassini spacecraft's (launched in 1997) UVIS package.
NOZOMI's ultraviolet (UV) instruments consisted of two parts: 1) a grating spectrometer for broadband hyperspectral imaging with a resolution of ~3 nm, and 2) an absorption cell photometer capable of discriminating H versus D emission at 121.567 nm and 121.534 nm nominal wavelengths with a miniscule 0.033 nm offset.
Because a dispersive spectrometer capable of resolving these lines is too large and heavy for space travel, satellite-based measurements rely on compact gas absorption-cell photometers to quantitatively discriminate the spectral fingerprints of H and D.
A better absorption cell
A hydrogen-deuterium absorption cell photometer is simply a photomultiplier tube (PMT) sitting behind a narrow bandpass filter and two gas cells—one containing hydrogen gas (H2) and the other deuterium gas (D2). When a tungsten filament in either gas cell is heated, H2 or D2 is thermally split into H or D atoms, "turning on" the corresponding isotope-specific resonant atomic absorption at the expense of the molecular absorption. By measuring attenuation of transmitted light when turning on one cell at a time, the H versus D emission can be quantified.
Initial development and characterization of the absorption cell photometer system for the NOZOMI mission was described by Kawahara et al., with a more complete instrument build published later.3,4 While the gas absorption cell photometer concept dates back to the 1960s, there are a wide variety of implementations.5 Key considerations are a narrow field of view for hyperspectral imaging in space, as well as good rejection of out-of-band light, most critically atomic oxygen emission at 130.4 nm.
Bertaux and colleagues used a concave holographic grating focused on a pinhole to simultaneously restrict field of view and bandwidth, while the Cassini instrument relies on a baffled tube and an oxygen gas cell as a spectral notch filter.6 In either case, quantification of the spectral response is needed to avoid recording spurious data or potentially destroying the detector with a bright out-of-band source.
Since gas absorption cells work as electrically driven narrowband attenuators, verifying their function, accuracy, and spectral bandpass during development and before spaceflight requires careful calibration—most critically a measure of gas-cell heater current versus transmission. Thus, there is a need for an accurate and stable ground support spectrometer-in this case, the McPherson Model 209 VUV spectrometer (see Fig. 1). High spectral accuracy, repeatability, and measurement stability are critical during development and testing, as the satellite-mounted photometer can necessarily only be as accurate as the tools used to test the absorption-cell response.
Single- versus dual-beam measurement
Most commercial ultraviolet-to-visible (UV-VIS) absorption spectrometers use a dual-beam measurement to quantify absorption and scattering by a sample. In this case, light from a single source is split into a sample and reference arm, and the ratio of the two yields a transmission value. This method is generally best where possible, as the differential measurement removes errors caused by source and detector drift.
However, single-beam measurement—where transmission is measured by moving the sample into and out of the beam path—has advantages as well. First, a single detector and optical train is needed, which minimizes size and cost—particularly important for high-vacuum instruments. In addition, a single beam maximizes signal throughput where strongly absorbing samples or weak sources may be an issue.
McPherson manufactures both single- and double-beam instruments for VUV spectrophotometry, although in this particular H/D case, the need for high throughput over a relatively narrow bandwidth makes a single-beam system the best choice.
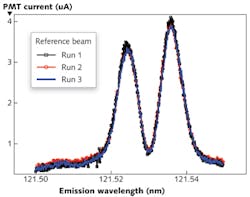
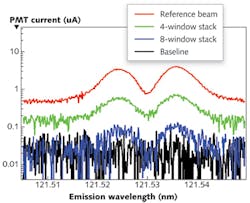
Single-beam measurements, however, do bring their own challenges: due to the lack of reliable VUV absorption standards, extremely stable and repeatable measurements of baseline and reference values are essential to making accurate measurements. For Lyman-α emission from a Hamamatsu deuterium lamp measured with the McPherson 209 scanning monochromator, the system is powered down, purged, and re-pumped for 45 minutes between each measurement. In less than an hour, the entire system is ready for more measurements with repeatable power and minimal baseline and source drift (see Fig. 2).Experiments used stacks of four and eight MgF2 windows to attenuate the beam. For the measured and calculated values, the baseline caused by detector dark current is measured separately and subtracted from reference and sample measurements. Counts are then summed over the hydrogen Lyman-α peak to generate a single value for each measurement (see Table 1).
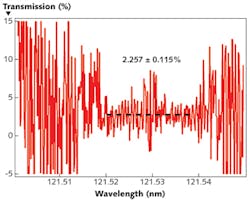
Unfortunately, the signal-to-noise ratio where the lamp is dim can be extremely low, as fluctuations due to noise dominate. Fitting to the high-signal region yields a transmission value that agrees with the table data shown previously. However, as one moves away from the peaks of the doublet, signal-to-noise drops off significantly-an unavoidable consequence of carrying out transmission measurements with an emission-line light source rather than a broad continuum.
Furthermore, we do not expect any sharp features in the absorption spectrum of MgF2 samples over such a small bandwidth (wavelength = 121.50–121.55 nm), which is to say that MgF2 absorbs and scatters light emitted from deuterium as readily as that emitted from hydrogen. When quantifying the function of an actual gas cell, this will certainly not be the case.
High spectral resolution, accuracy, and repeatability make McPherson spectrometers an excellent choice for calibrating gas cell photometers over several decades of transmission. With the appropriate light source, grating, and detector, it should be possible to extend this H/D measurement method to build and characterize absorption spectrum photometers for remote detection and quantification of other isotope mixtures.
REFERENCES
1. L. Esposito et al., "The Cassini ultraviolet imaging spectrograph investigation" in The Cassini-Huygens Mission (Springer, 2004), 299–361.
2. M. Taguchi et al., Earth, Planets and Space, 52, 1, 49–60 (2000).
3. T. Kawahara et al., Tohoku Geophysics J., 5, 34, 2, 35–54 (Tohoku University, 1993).
4. T. Kawahara et al., Appl. Opt., 36, 10, 2229–2237 (1997).
5. D. C. Morton and J. D. Purcell, Planet. Space Sci., 9, 8, 455–458 (1962).
6. J. Bertaux et al., Science, 225, 4658, 174–176 (1984).
Adam J. Wise is director of research & development at McPherson, 7-A Stuart Road, Chelmsford, MA 01824-4107; e-mail: [email protected]; www.mcphersoninc.com.