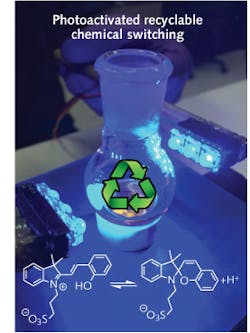
Visible light eliminates molecular switching waste
Chemically activated molecular switches and machines are used in applications ranging from drug delivery to molecular actuators. Unfortunately, these molecular switches require consumable reagents that create waste products that hinder long-term function. In response to this challenge, researchers at Dartmouth College (Hanover, NH) have developed a molecular switch using a visible-light-activated photoacid that produces essentially no waste products and is functional for more than 100 switching cycles—an unprecedented result that is also applicable to aqueous environments and hence a variety of biological applications.
To perform waste-free switching, a merocyanine-based photoacid was irradiated using a hyper-violet LED, resulting in the formation of a spiropyran molecule and the release of an acid to the environment. This change in pH was then used to activate a hydrazone-based switch; the molecule was induced to rotate 180° around an axle, leading to a change in its shape and photophysical properties. The recyclability of this process stems from the fact that the spiropyran molecule is not thermodynamically stable and so with time it picks the proton back from the hydrazone switch to reform the merocyanine compound and reset the whole switching process. Because this process is highly efficient, it can be cycled more than 100 times with no apparent fatigue. Reference: Luke A. Tatum et al., J. Amer. Chem. Soc. 136, 17438–17441 (Dec. 4, 2014). For more information, contact Ivan Aprahamian at [email protected]