Tissue Engineering: 3D laser printing of live human cells promising for testing, transplantation
Researchers in Germany have adapted a laser forward transfer method for printing living cells. The approach provides resolution and cell densities sufficient for tissue formation. The technique has been shown to enable production of 3D cell constructs using live human cells, which could obsolete animal testing of chemicals, pharmaceuticals, and cosmetics. Ultimately, it could also allow the manufacture of complete functional replacement organs for transplantation.
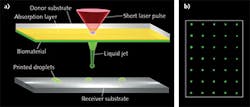
Boris Chichkov, Urs Zywietz, and Lothar Koch of Laser Zentrum Hannover e.V. coat a glass slide or transparent ribbon with one layer each of a laser-absorbing material and a biomaterial—typically a hydrogel with embedded cells (see Fig. 1). With the glass slide mounted upside down, they direct 10 ns pulses of 1064 nm laser light through the slide into the absorption layer, which evaporates in the focal spot. The vapor pressure propels the subjacent biomaterial forward and deposits it as a droplet onto a surface under the slide. By moving the slide and the laser beam, they can print any 2D or 3D patterns desired—layer by layer.
The process does not harm cells or affect stem cells' differentiation behavior or potential; in fact, stem cells printed in patterns differentiate within the patterns toward different tissue types (see Fig. 2).1,2 As well, the team observed directed migration of adipose-derived stem cells toward endothelial cells and their interactions in establishing blood vessels.3To form 3D skin tissue constructs, the team printed fibroblast and keratinocyte cells layer by layer. They have proven skin tissue formation by visualizing intercellular junctions and verifying their functionality,4 and they have observed basal lamina formation. Implanted in mice, the printed skin constructs show an ingrowth of blood vessels and differentiation of the epidermal keratinocytes.5
Next, the team will print bacteria and microorganisms.
REFERENCES
1. L. Koch et al., Tissue Eng. Part C Methods, 16, 5, 847–854 (2010); http://dx.doi.org/10.1089/ten.tec.2009.0397.
2. M. Gruene et al., Biofabrication, 3, 015005 (2011); http://dx.doi.org/10.1088/1758-5082/3/1/015005.
3. M. Gruene et al., Tissue Eng. Part C Methods, 17, 973–982 (2011); http://dx.doi.org/10.1089/ten.tec.2011.0185.
4. L. Koch et al., Biotechnol. Bioeng., 109, 7, 1855–1863 (2012); http://dx.doi.org/10.1002/bit.24455.
5. S. Michael et al., PLoS One, 8, 3, e57741 (2013); http://dx.doi.org/10.1371/journal.pone.0057741.

Barbara Gefvert | Editor-in-Chief, BioOptics World (2008-2020)
Barbara G. Gefvert has been a science and technology editor and writer since 1987, and served as editor in chief on multiple publications, including Sensors magazine for nearly a decade.