Neurology/Alzheimer's: Laser-based eye test for early detection of Alzheimer's
PAUL HARTUNG
Alzheimer's Disease International (London, England) estimates that the number of people living with dementia worldwide (nearly 44 million in 2014) will almost double by 2030 and more than triple by 2050. The global cost of dementia was estimated at $604 billion in 2010.1 Many clinical trials show that early intervention and treatment are the only way to slow and, perhaps, reverse the progression of the disease. Today, Alzheimer's disease (AD) is diagnosed using an expensive and lengthy process of elimination of other possible causes: it takes nearly two years for patients to be evaluated and another year to make a clear diagnosis.2 By the time a diagnosis of probable AD can be made, typically 40-50% of brain function is lost.
There has been a major effort to develop methods of measuring and monitoring beta amyloid (Aβ) deposition, a hallmark of AD. Cerebrospinal fluid (CSF) markers have been developed, based on the finding that as Aβ levels increase in the brain, they decrease in CSF. The fact that lumbar puncture is required has limited the adoption. More recently, positron emission tomography (PET) amyloid imaging of the brain has been approved to rule out AD. If a patient has a negative scan, it is considered unlikely that AD is the cause of the dementia. The high cost (approximately $5000) of this procedure, the limited availability of PET scanners, and the need for injection of a radio-pharmaceutical have limited the adoption of PET in clinical practice.
Researchers have long established the close connection between the eye and the brain. Based on a breakthrough discovery that Aβ grows in the lens of the eye, and that this parallels the growth in the brain, Cognoptix is developing a noninvasive, low-cost test to aid in the diagnosis of AD at the point of care.
A novel eye scan
Over the past decade, the understanding of the AD process has grown significantly. AD is confirmed at autopsy by plaques and tangles formed in the brain. Plaque is made up of Aβ 1-40 and 1-42. Increases in Aβ levels begin at the earliest stages of the disease process, years before symptoms arise. Studies have shown that amyloid is building up in the brain up to 30 years before clinical signs of dementia appear.3-4 When an individual starts to show signs of cognitive impairment, it is called mild cognitive impairment (MCI). MCI, sometimes only temporary, can occur because of a number of causes, including depression and anxiety.5
Goldstein et al. made the discovery that Aβ aggregates in the lens of the eye, and at a late stage in the disease, exhibits itself in the form of a supranuclear cataract. In age-matched controls, which included patients with Parkinson's disease, frontotemporal dementia, and other neurodegenerative diseases, the Aβ pathology was not found.6
Cognoptix's SAPPHIRE II system (see Fig. 1) is a novel drug/device combination for early AD pathology detection. An ophthalmic ointment containing an amyloid-binding ligand is administered to the inner eyelid the day before the procedure. The lens of the eye is then scanned with a low-power laser. This causes the ligand/amyloid complex to fluoresce. The systems measures the fluorescence data to determine how much amyloid there is in the eye.
The Cognoptix eye test takes less than five minutes, and results are generated immediately. It is relatively inexpensive-10 times less expensive than any of the current diagnostic procedures, and a lot less expensive than imaging amyloid in the brain. The test is safe, as there are no toxicity issues, and easy to use, as it does not require a blood draw or a spinal tap.
A scan can be completed in approximately one second without dilation of the pupil. The patient sits in a chair and the patient uses a chin and forehead rest to steady themselves while they focus on a fixation target. The operator aligns the instrument to the patient using a joystick and, when ready, starts a scan. A scan takes about a second to perform, so multiple scans may be performed in under five minutes. The instrument automatically finds the region of interest in the supranucleus of the lens of the eye, collects the data, and computes a score called the fluorescence uptake value (FUV).
The test is ideally suited for integration into primary care setting. Aids, technicians, and nurses may be trained to perform the test. The test is easy to administer, and the results are automatically generated onsite. Initially, we see it being utilized as a diagnostic aid for patients who are starting to show signs and symptoms of AD. Since the pathology of AD begins years before symptoms arise, we envision a future where our test may be used as a screening test starting at age 45-50.
Neurologists may be early adopters of the test, as they are currently involved in Alzheimer's diagnoses, and they are looking for more cost-effective and noninvasive methods to test for Alzheimer's pathology. Ophthalmologists are also looking to expand their practices and could incorporate the SAPPHIRE exam as part of their comprehensive eye exams in the future.
One of the reasons this test is so important is that many patients are being treated by general practitioners with really insufficient workup. About 40% of the Alzheimer's diagnoses are made in the primary care setting, with very little data.
The Cognoptix test may lead to better patient outcomes. If we can integrate clinical evaluation of a patient and combine that with the FUV data, this should allow early treatment before significant cognitive and functional damage occur. Early diagnosis, early intervention, and early treatment are the keys to slowing the progression of AD.
Studies have begun to demonstrate the benefits of early intervention. Recently, a Biogen Inc. drug called aducanumab that targets plaque buildup not only reduced amyloid plaque levels in the brain, but slowed cognitive decline in patients with early and mild forms of AD in a Phase 1b study. Biogen has announced that they are now moving into Phase 3.
Principle of operation
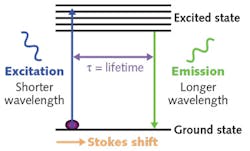
Figure 2 demonstrates the principle of the Cognoptix technology. It is based on the concept of excitation of a fluorescent Aftobetin ligand that is applied topically to the eye. A low-power pulsed laser (shown in blue) is used to excite the ligand molecule, which in turn emits light (shown in green) at a different wavelength based on the Stokes shift effect.Preclinical studies demonstrated the ability to deliver ligand to the lens via a topical ocular administration with no safety issues, and also validated the separation of signals of amyloid-bound ligand and unbound ligands in vitro. Equally important, the studies demonstrated in vivo linear and planar scanning capability for characterization of lens anatomy and ligand distribution.
Clinical studies
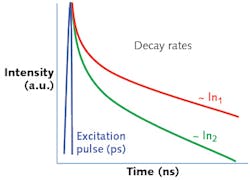
In 2012, Cognoptix published results of a 10-patient proof-of-concept clinical study of the SAPPHIRE II System. The study included 5 patients with AD and 5 "normal" individuals. The SAPPHIRE II System was able to effectively separate the average of fluorescent intensity in the two groups.7
In 2014, Cognoptix published a 40-person Phase 2 clinical trial conducted at four sites on patients clinically diagnosed with probable AD via a rigorous neuropsychological and imaging workup, and age-matched healthy controls, outstanding results of 85% sensitivity, and 95% specificity were achieved in predicting probable AD. All of the participants in the trial were tested with PET amyloid imaging of the brain, and the SAPPHIRE II test achieved tighter correlation to clinical diagnosis than PET imaging, which achieved 80% sensitivity and 80% specificity by visual read.8-9
Cognoptix is now planning a pivotal Phase 3 trial as the final stage in clinical development to support FDA pre-market approval (PMA) of the SAPPHIRE II system for clinical use. Manufacturing of the device and the ophthalmic ointment are being scaled up.
The impact
The Cognoptix test is positioned to change the way Alzheimer's patients are identified and treated. There is a significant need today for a low-cost, point-of-care test to help differentiate people who have Alzheimer's pathology from those who don't to more effectively manage the treatment and care of those with the disease, and to avoid costly diagnostic procedures. The impact of such a test could be profound for the patients, their families, and caregivers.
REFERENCES
1. M. Prince, E. Albanese, M. Guerchet, and M. Prina, World Alzheimer Report 2014; see http://bit.ly/1o2gvVX.
2. C. R. Jack, Alzheimers Dement., 7, 3, 257-262 (May 2011).
3. P. J. Visser et al., JAMA, 313, 19, 1924-1938 (May 19, 2015).
4. P. J. Visser et al., JAMA, 313, 19, 1939-1949 (May 19, 2015).
5. M. S. Albert et al., Alzheimers Dement., 7, 3, 270-279 (May 2011).
6. L. E. Goldstein et al., Lancet, 361, 9365, 1258-1265 (2003).
7. C. Kerbage, C. H. Sadowsky, D. Jennings, G. D. Cagle, and P. D. Hartung, Front. Neurol., 4, 62, 1-9 (May 2013).
8. C. Kerbage et al., Am. J. Alzheimers Dis. Other Demen., doi:10.1177/1533317513520214 (Feb. 13, 2014).
9. C. H. Sadowsky et al., JSM Alzheimer's Dis. Related Dementia, 1, 2, 1008, 1-5 (Oct. 2014).
Paul Hartung is an independent consultant for and shareholder at Cognoptix, Acton, MA; e-mail: [email protected];www.cognoptix.com.