3D Microscopy: Single-objective SPIM simplifies super-resolution 3D cell imaging
A simplified super-resolution microscopy method can identify single proteins anywhere within a cell, and allows assessment of cellular organization in 3D.1
Recent advances have enabled super-resolution imaging of biological samples in 3D for extended periods without damaging the sample. This means that the activity of single proteins can be followed within individual cells, providing new insight into protein function and, importantly, how protein dysregulation can lead to disease. Unfortunately, these techniques are complicated and expensive, and most of those that enable single-molecule imaging capture images only within the first micrometer of the coverslip-whereas a typical human skin cell is 30 μm thick.
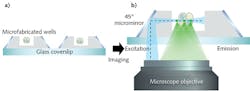
And while selective plane illumination microscopy (SPIM) enables 3D super-resolution imaging of thicker samples at a single-cell level, it requires a two-objective system and sample holder that is incompatible with standard microscopes. But an updated approach, called single-objective SPIM (soSPIM), requires just one objective. Developed by associate professor Virgile Viasnoff of the Mechanobiology Institute (MBI) at the National University of Singapore, soSPIM uses an array of micromirrored wells: Each mirror is inclined at precisely 45°, and serves as both a means to direct the excitation beam and to hold the sample. Together with a beam-steering add-on unit, these micromirrors enable the excitation beam and the fluorescence signal to pass through a single standard objective lens.
Compatible with standard inverted microscopes and high-numerical-aperture immersion objective lenses, soSPIM has exhibited fast response, good sectioning capability for 3D imaging of whole cells up to 30 μm above the coverslip, and the ability to identify single proteins deep within cells.
1. R. Galland et al., Nat. Methods, 12, 641-644 (2015); doi:10.1038/nmeth.3402.

Barbara Gefvert | Editor-in-Chief, BioOptics World (2008-2020)
Barbara G. Gefvert has been a science and technology editor and writer since 1987, and served as editor in chief on multiple publications, including Sensors magazine for nearly a decade.