Diamond Anvil Cells: Laser heating of samples at high pressure: 50 years
WILLIAM A. BASSETT
Diamond anvil cells are capable of subjecting samples to very high pressures so that analytical techniques can be used to study the effects of high pressure on their properties.1 Although there were already a number of large-volume presses capable of achieving high pressures, the diamond anvils' small size and extreme hardness made it possible to achieve very high pressures.
Fortunately, diamonds are also transparent to a large portion of the electromagnetic spectrum, making it possible to visually observe the sample, as well as to use x-rays, ultraviolet (UV), and infrared (IR) radiation. Optical microscopes capable of observing micrometer-size samples easily provide detailed images of samples as pressure is applied. At the same time that diamond anvil cells were being developed, great advances were being made in instrumentation for analyzing smaller and smaller samples by x-ray, UV, visible, and IR beams. Most of those improvements were taking place at synchrotron light sources.
It was only natural that early users of diamond anvil cells would wish to subject their samples to simultaneous high pressures and high temperatures. The earliest approach was to wind wires around the supports for the anvils—a technique still in use today. Temperatures, however, were limited to around 1200°C by the stability of the diamond anvils, which, although very durable, are thermodynamically metastable. So, there was a need for some method that would be able to heat samples to much higher temperatures without affecting the anvils or the pressure.
Genesis of laser heating with diamond anvils
At lunch one day in 1966, my close friend and colleague Taro Takahashi was speculating on ways such high temperatures might be possible when he came up with the suggestion of using a laser beam focused through one of the diamond anvils that would directly heat the sample at the same time that it was under pressure. We both immediately recognized the possibilities, especially if it could be done without damaging the anvils.
Lasers were still very new at that time, and the pulsed ruby laser used for etching and drilling was the best known. Its 694.3 nm red light could easily pass through one of the diamond anvils to the sample. So, our first attempt at laser heating employed a pulsed ruby laser purchased from Hughes. With it, we were able to convert graphite to diamond once we realized that the graphite needed to be small particles suspended in a transparent soft solid such as sodium chloride (NaCl).
We soon found we could drive other important phase transitions. However, the single-shot ruby laser was agonizingly slow for yielding useable quantities of high-pressure phases. The next step was to switch to one of the newer lasers that could put out a continuous beam at a wavelength for which diamond is transparent. Such a laser turned out to be neodymium (Nd)-doped yttrium aluminum garnet (Nd:YAG) with a wavelength of 1064 nm and a watts-level output power. We acquired a Quantronix series 100 Nd:YAG laser capable of 18 W continuous-wave (CW) with a Q-switch capable of producing extremely intense pulses at up to kilohertz rates.
Of particular interest to us as geologists was the ability to drive such transitions in silicates. My student, Li-chung Ming, was able to show that the mineral fayalite breaks down to mixed oxides of iron and silicon at pressures higher than the stability field of its spinel phase.2 We could raster the focal spot of the pulsed beam across the high-pressure portion of our sample until all of it was converted. And we were able to do this without damaging the diamond anvils—most of the time.
The exceptional clarity of the gem diamonds, their good thermal conductivity, the strongly convergent laser light, and dark-colored samples surrounded by colorless medium all helped. I once measured a temperature on the order of 7000 K—an impressive temperature, but not very useful when one considers that the duration was only about 16 ns and the temperature was more an estimate than a measurement.
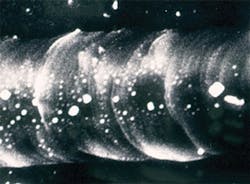
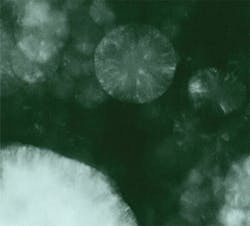
However, results of such an intense beam could be dramatic. Figures 1 and 2 show what can happen when a dark sample is in contact with an anvil face or there is a defect such as a crack. The medium between the anvil faces in Figs. 1 and 2 was potassium bromide (KBr) with pressure increasing from left to right, from the stability field for graphite (opaque) to the stability field for diamond (transparent). The smooth surface of the transparent part of the groove, its raised edges, and embedded KBr provided convincing evidence that the diamond had melted.3Of course, overcoming kinetic barriers was just one interesting application of the high temperatures possible with laser heating. Mapping phases on a pressure-temperature (P-T) diagram and measuring full pressure, volume, and temperature (PVT) equations of state were clearly very desirable objectives as well. But that required a uniform temperature in the volume of sample to be analyzed, a temperature that remained constant long enough to allow collection of data, and the ability to make accurate measurements of temperature in that volume and for that duration. However, such control was not the laser's strong point, and not all samples were dark enough to absorb the laser energy.
The next step was to use the 18 W CW output from the Nd:YAG laser. Fortunately, all of the sample temperatures possible with lasers but not possible with resistance heaters are incandescent. That makes it possible to determine temperature by spectroradiometry—the use of the spectrum of blackbody emission from the sample. Spectrometers being developed at that time could collect an entire spectrum on a photodiode array from near-IR to UV, making it possible to accurately fit a spectrum to published blackbody spectra and determine the temperature of an incandescent sample.Unless a sample was dark-colored, it was usually necessary to mix a transparent medium with an opaque powdered sample and to mix an opaque powder with a transparent sample. Another problem with the blackbody method of measuring temperature is that few samples are as black as graphite, meaning that a correction for a lower emissivity should be made. An even more serious problem with the laser heating was the steep axial and radial temperature gradients that would fall within an x-ray beam being used to probe a sample's properties. Ways of overcoming this problem are described in the next section.
Double-sided laser heating
Researchers at the Geophysical Laboratory of the Carnegie Institution of Washington (Washington, DC), in collaboration with beamline scientists at the Advanced Photon Source (APS) at Argonne National Laboratory (Argonne, IL), devised ingenious ways of meeting this challenge.5 They designed a double-sided, multimode laser heating system (see Figs. 4 and 5) consisting of two CW neodymium:yttrium lithium fluoride (Nd:YLF) lasers, which were found to be more stable than the Nd:YAG lasers used earlier.
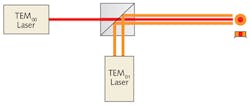
One laser was configured to produce a TEM00 mode and the other set for TEM01 mode. The two beams (which have a 1053 nm wavelength) were combined so that the TEM00 beam filled in the center of the TEM01 donut mode, producing a 100 W combined beam with a flat-top intensity profile (see Fig. 5) that could be balanced by optically controlling the intensities of the two laser beams. They then split the combined beam into two beams, with one directed to one side of the sample and the other directed to the other side (see Fig. 4).
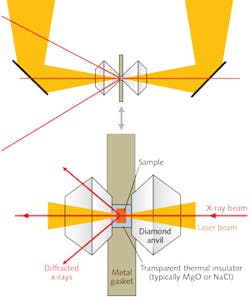
Samples must always be thermally insulated from the anvils by a transparent inert material such as magnesium oxide (MgO) or NaCl. With this system, the researchers were able to heat a sample volume 10–25 μm across and 10–50 μm thick to temperatures in the range of 1200 to 4000 K and pressures up to 160 GPa. They could image the sample on a CCD detector capable of providing spectroradiometric temperatures at many points within the image. The extraordinarily small x-ray beams available at synchrotron sources (3–10 μm) could easily fit within a sample this size. Additional stability was developed by means of feedback-stabilization laser circuitry. The radial, axial, and temporal variations in their sample could be limited to <50 K.The double-sided laser-heating systems developed at Sectors 13 and 16 at the APS, which are capable of accurate determination of temperatures up to 4000 K in samples at pressures as high as 160 GPa, continue to yield a great deal of valuable data for research in a variety of scientific disciplines. Geophysics in particular has benefited, as these capabilities have opened the way to characterization of properties of materials that play a key role in interpreting geophysical observations of planetary interiors.
REFERENCES
1. W. A. Bassett, High Pressure Res., 29, 163–186 (2009).
2. W. A. Bassett and L. C. Ming, Phys. Earth Planet. Interiors, 6, 154–160 (1972).
3. J. S. Gold, W. A. Bassett, M. S. Weathers, and J. M. Bird, Science, 225, 921–922 (1984).
4. W. A. Bassett and M. S. Weathers, "Fullerene structures produced from melted diamond at high pressure by laser heating," Proc. AIRAPT, High-Pressure Science and Technology – 1993, AIP Conference Proceedings 309 Part 1, 651–653 (1994).
5. G. Shen, M. L. Rivers, Y. Wang, and S. R. Sutton, Rev. Sci. Instrum., 72, 1273–1282 (2001).
William A. Bassett is professor emeritus in the Department of Earth and Atmospheric Sciences at Cornell University, Ithaca, NY; e-mail: [email protected]; www.eas.cornell.edu.