Bioanalytics/Illumination: Just-right light - Inherently adaptive for application-specific needs
The economic, performance, environmental, and health advantages of solid-state light sources—including output stability, power efficiency, and elimination of hazardous substances such as mercury1-3—are driving the displacement of incandescent-lamp sources with solid-state light engines in bioanalytical applications. Less well appreciated is the extent to which solid-state sources facilitate application-specific customization and illumination optimization. This article presents three examples—among many opportunities—to illustrate this paradigm.
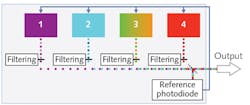
A light engine is an illumination source consisting of an integrated array of LEDs, lasers, and luminescent light pipes (solid-state sources offering electronic control characteristics similar to LEDs, but with greater spectral bandwidth; see Fig. 1). The number of sources in the array, and the wavelength, bandpass, optical power, and mode of operation of each, can be configured according to application requirements.
Spectral output customization
Fluorescence in situ hybridization (FISH) is the cornerstone of cytogenetic analysis.4 Typically, specimens are interrogated with multiple spectrally distinct probes, allowing simultaneous visualization of variant and control nucleotide sequences.
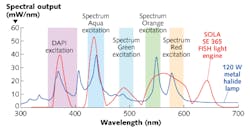
The ideal light source for FISH should provide output that is spectrally optimized relative to the excitation characteristics of the probes, and deliver sufficient intensity to generate fluorescence from weak hybridization signals. In FISH, as in other multiplex fluorescence imaging applications, it is standard practice to use approximately 30 nm excitation bandwidths evenly distributed across the visible spectrum and its adjacent fringes (from approximately 350 to 650 nm), allowing discrete detection of five or six nucleotide sequences.
Incandescent illumination sources such as metal halide lamps and mercury arc lamps have an invariant visible output spectra consisting of a triplet of intense peaks (365, 405, and 436 nm) separated by a gap of about 100 nm from another intense doublet (546 and 578 nm; see Fig. 2). The gap spans the optimal excitation region for SpectrumGreen (Abbott Molecular)-labeled FISH probes, and other important labels such as fluorescein isothiocyanate (FITC) and green fluorescent protein (GFP).In contrast, the spectral output of a solid-state light engine can be configured based on FISH-specific excitation requirements. Although the total output power of the light engine and the metal halide lamp are about the same, the light engine's output is concentrated in wavelength regions wherein it can be absorbed and converted into detectable fluorescence—and not in regions where it is irrelevant to the application and potentially damaging to the specimen (e.g., <350 nm). Thus, in the example shown in Fig. 2, the light engine provides stronger excitation than the metal halide lamp in four out of five detection channels.
The advantage is even greater at wavelengths >600 nm, which are used for excitation of Cy5 and SpectrumFRed (far red)-labeled FISH probes (Abbott Molecular). There, metal halide and mercury arc lamps generate some of their lowest optical power, while the light engine affords a significant spectral peak.
Fast wavelength switching
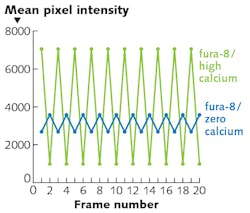
Ratio imaging of intracellular calcium has long been an important technique in cell biology, neuroscience, and related fields.5 Excitation ratio imaging compensates for variations of indicator dye concentration—both within and between cells—that might otherwise be interpreted as calcium level changes. The preferred indicator dyes for ratio imaging of calcium ions (Ca2+) are typically fura-2 and the recently developed red-shifted analog fura-8 (AAT Bioquest, Inc.).
Excitation ratio imaging is conventionally implemented using a white light source in combination with mechanically alternated filters to select the desired excitation wavelengths (340 and 380 nm for fura-2, and 360 and 400 nm for fura-8). A solid-state light engine can generate these outputs from two filtered LED sources that can be electronically selected without mechanical intervention. Because source-switching is accomplished without moving parts, alternation of excitation wavelengths (see Fig. 3) can be more than 1000 times faster (mechanical switching ~50 ms vs. electronic switching ~10 μs) and is less susceptible to inter-cycle variability than mechanical methods. In turn, this allows higher-speed data acquisition, providing increased temporal resolution for recording elementary processes in cell physiology.Among the many other applications that can benefit from the electronic switching of solid-state light sources is high-speed color imaging in both fluorescence and transmitted light microscopy.2
Multimode output optimization
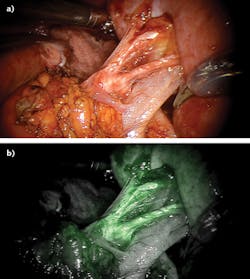
In intravital imaging, the increased complexity of the specimen relative to cell cultures and tissue sections frequently creates a need to combine imaging modalities with different spatial resolution and tissue penetration characteristics. A good example of this requirement is found in robotic-assisted laparoscopic surgery.
In such operations, the surgeon views the operative field by reflectance endoscopy using white light illumination. Superimposition of other imaging modalities provides enriched data for additional surgical guidance. For example, near-infrared (near-IR) fluorescence imaging of the vasculature provides identification of vessels to be clamped in the vicinity of excision sites and also supports differentiation of tumors from surrounding tissue (see Fig. 4).6 Alternating observation of the same field of view by these two optical imaging modalities requires integrated light sources.Customized light engines for this application consist of four visible-wavelength sources that combine to generate white light and a near-IR laser for fluorescence excitation. Both outputs are directed to the operative field through the same endoscope. The relative power outputs of the four visible-range sources are adjustable, allowing optimization of the color temperature and color rendering index of the white-light output.
Equally important is the capacity of the light engine to generate the substantially higher power levels required for illumination of a macroscopic subject compared to those typically used for imaging microscopic specimens. Here, solid-state light engines are engineered to deliver optical power outputs rivaling that of the 300 W Xenon lamp alongside watts of near-IR laser light for fluorescence excitation via a single optical train that is both spectrally and temporally well controlled.
And more
FISH, ratio imaging, and intravital imaging are representative examples of a wide range of applications for which solid-state sources can be easily leveraged to maximize performance.
Whereas traditional outputs involve physically invariant spectral distribution, solid-state illumination is highly amenable to additive assembly. And whereas traditional outputs are commonly created by selective blocking and attenuation of superfluous outputs, the modern, solid-state approach assembles component sources within an inherently efficient, integrated control framework according to application requirements.
While reasons of convenience (such as precision electronic control of output intensity, color, and timing) or avoidance of environmental contaminants have compelled many people to switch to solid-state light engines, the ability of this new generation of sources to better address the needs of particular bioanalytical applications is becoming an important reason as well. In any case, the benefits of solid-state engines are making a practical difference, as an increasing number of system designers and end users can attest.
REFERENCES
1. T. R. Baird, D. Kaufman, and C. M. Brown, J. Biomol. Tech., 25, 48-53 (2014).
2. S. Sato and V. N. Murthy, Cold Spring Harb. Protoc., doi:10.1101/pdb.top072306 (2012).
3. M. Macka, T. Piasecki, and P. K. Dasgupta, Annu. Rev. Anal. Chem., 7, 183-207 (2014).
4. J. T. Mascarello et al., Genet. Med., 13, 667-675 (2011).
5. N O'Connor and R. B. Silver, Methods Cell Biol., 114, 387-406 (2013).
6. S. Tobis et al., J Urol., 186, 47-52 (2011).
Iain Johnson | Director of Technical Support, Lumencor
Iain Johnson is Director of Technical Support at Lumencor (Beaverton, OR).