Healthcare: Laser-based point-of-care testing and biomodulation therapy have a bright future
Optical technologies have been instrumental in healthcare for hundreds of years, starting with the invention of eyeglasses in the late 13th century in Italy.1 In the 17th century, Robert Hooke and Antonj van Leeuwenhoek went on to revolutionize our understanding of medical science when they became the first people to use a microscope to investigate cellular structures.2 As a result, optics singlehandedly facilitated the development of microbiology, which led to the modern germ theory of disease proposed by Louis Pasteur 200 years later. Today, optics and photonics are so intertwined with modern healthcare that an entire chapter of the 2013 National Research Council (NRC) report on optics and photonics, chaired by Paul McManamon and Alan E. Willner, was dedicated to “Health and Medicine.”3
The laser also has a long history of application in healthcare, dating back to 1964 when a ruby laser was used to treat melanomas and thyroidal carcinomas.4 Today, lasers are used in medical applications ranging from elective aesthetic procedures, such as hair and tattoo removal, to necessary surgical procedures using endoscopic laser ablation.5 In addition to therapeutics and surgery, lasers are used regularly for in situ diagnostics. As a result, lasers have become an integral tool in nearly every field of healthcare including dermatology, oncology, urology, and many others.6
Medical laser applications have historically been focused on ablation, cutting, and cauterization, while recently there is an emerging trend of lower-power nondestructive uses. This article will focus on two such categories of lower-power medical laser applications that are poised to be critical in shaping the future of photonics in healthcare: point-of-care (POC) testing and photobiomodulation therapy (PBMT).
Point-of-care testing
Traditionally, medical testing relies on a multistage process wherein a medical professional collects a sample (such as blood, tissue, saliva, or urine), which is sent to a laboratory to be assayed and tested. Several hours to days later, the results are then reported back to the clinician, enabling an informed decision to be made about the next steps in a patient care plan. Unfortunately, the time delay associated with traditional laboratory testing not only serves as an inconvenience in light of modern on-demand expectations, but in many cases leads to adverse patient outcomes. To mitigate these delays, the healthcare industry has been rapidly adopting POC testing, providing clinicians real-time, laboratory-quality diagnostic results within minutes.
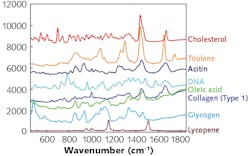
Vibrational spectroscopy, and Raman spectroscopy in particular, is one of the most promising methodologies for enabling the next generation of in vivo and ex vivo POC testing. Raman spectroscopy is a laser-based technique for determining molecular structure. This is done by exciting a sample with a wavelength-stabilized laser source (typically a 785 nm, volume Bragg grating-stabilized diode) and measuring the frequency shift in the inelastically scattered photons. Since the energy difference between the incident and scattered photons is directly related to the vibrational mode structure of the sample, the Raman spectrum serves as a unique molecular fingerprint (see Fig. 1).
Raman spectroscopy has been shown in clinical trials to be a useful tool for the detection of a wide range of diseases, including cancers. One of the most exciting demonstrations was by Jonathan Horsnell, a surgeon at Gloucestershire Royal Hospital (Gloucester, England). He successfully classified cancerous and benign axillary lymph nodes during breast-cancer surgery using a portable Raman system.7, 8 This technology has the potential to save countless lives in the future by enabling surgeons to better identify tumor margins during an operation without having to wait for post-surgical histopathology.
Traditional Raman spectroscopy suffers from two primary drawbacks: signal strength and depth penetration. However, there are several enhancement techniques that significantly mitigate these issues. Surface-enhanced Raman Spectroscopy (SERS) is a signal-enhancement technique that uses local surface-plasmon resonance via nanofabricated materials (typically gold or silver) to increase the Raman scattering efficiency by six to eight orders of magnitude. SERS can be performed on either nanostructured substrates or colloidal nanoparticles, which makes it a viable option for both in vivo and ex vivo POC testing.
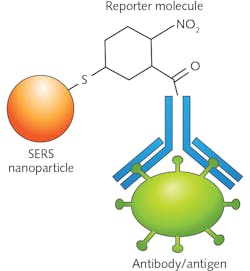
An additional benefit of using SERS is the ability to functionalize the substrate or nanoparticles with antibodies and capture molecules that can allow for selective binding of particular analytes of interest, as well as reporter molecules that can be engineered to have specific spectral features.9, 10 Figure 2 shows a schematic representation of a SERS nanoparticle that has been functionalized with a nitrogen dioxide (NO2) reporter with a strong Raman peak around 1300 cm-1 and an antibody to capture a specific antigen.A process known as spatially offset Raman spectroscopy (SORS), which was first pioneered by Pavel Matousek’s group at the Rutherford Appleton Laboratory (Didcot, England), provides a method for overcoming the depth-penetration issue.11 SORS uses a variable spatial offset between the excitation laser and the collection path to measure the Raman scattering deep inside the tissue, instead of the scatter from the surface. SORS has shown significant promise as a noninvasive method of identifying subdermal maladies such as bone disorders,12 calcifications, and breast cancer.13
Nick Stone’s group at the University of Exeter (Exeter, England) has further built upon Matousek’s work by combining these two approaches into a new hybridized technique called surface-enhanced spatially offset resonance Raman spectroscopy (SESORRS). In biomedical applications of SESORRS, SERS nanoparticles are first injected into tissue and then SORS is used to record the spectrum, providing signal enhancement deep below the surface of the skin.14 Recently, a group at the University of Strathclyde (Glasgow, Scotland), which included Duncan Graham, Karen Faulds, Fay Nicolson, and several others, demonstrated the ability to use SESORRS to image live breast-cancer tumors using a handheld instrument, showcasing the ability for SESORRS to be used as a true POC technology.15
It is important to remember that Raman spectroscopy is one of the many spectroscopic tools that are currently being investigated for POC testing. Other notable techniques being explored include infrared spectroscopy, fluorescence spectroscopy, and colorimetry. Most researchers in the field are of the opinion that, for POC testing to ever achieve a real-world version of the fictional tricorder from Star Trek, a multimodal approach incorporating several complementary technologies will be required.
Photobiomodulation therapy
It has long been understood that light-based therapy could be used for therapeutic treatment of certain conditions such as jaundice,16 but until recently the field of low-level light therapy (as PBMT was previously known) was tarnished by a wide range of unsubstantiated claims and “home cures.” However, over the past several years, a large number of repeatable medical studies in both animals and humans have begun to shed light on the underlying biophysical mechanism behind clinical applications of PBMT.17-19 In fact, as recently as August 2019, Praveen Arany from the University of Buffalo’s School of Dental Medicine published an article in Laser Focus World aptly titled “Phototherapy: Photobiomodulation therapy—easy to do, but difficult to get right” highlighting some of these issues.20
LEDs and other broadband light sources have been studied with regard to PBMT, but lasers are typically preferred for clinical applications due to the ability to better direct the photon flux, resulting in greater depth penetration. In PBMT, the exact laser wavelength needed is highly application-dependent, but noninvasive applications take advantage of the dip in tissue absorption between 600 and 1000 nm. Some of the clinical applications where PBMT shows promise are in the fields of diabetes, neural diseases, dermatology, and dentistry, and, perhaps the most interesting, the field of sports medicine.19
The reason why deep-tissue PBMT is so attractive for sports medicine is that light stimulates cytochrome c in cell mitochondria, resulting in increases in adenosine triphosphate (ATP), free nitric oxide (NO), and reactive oxygen species (ROS).21 ATP is responsible for energy transfer, NO vasodilation, and ROS inflammatory response, therefore leading to both pain relief and reduced recovery times. These photophysiological effects have been known for years, but previous attempts at using class IIIb lasers failed to provide adequate depth penetration for clinical effects in the subdermal tissue (see Fig. 3). Now that class IV near-infrared laser diodes have become readily available, the adoption of PBMT in sports medicine has skyrocketed.According to Andy Wood, Vice President of International Sales for LiteCure, LLC (New Castle, DE), “Pro sports teams have adopted LightForce [PBMT] lasers as part of their clinical toolbox. Most MLB, NFL, NHL, and NBA teams have at least one unit.” Wood went on to say that, “The English Premier League is also embracing laser therapy, with around 10 teams now using it.” Ed Ryan, Medical Director for U.S. Olympic Basketball, said, “Deep-tissue laser therapy is very effective at mitigating the painful sequelae of acute injuries, as well as enhancing the healing environment for both acute and chronic injuries.” As both prices and stigmas are reduced, it is likely that deep-tissue laser therapy will become more and more available to the general public.
Another truly cutting-edge example of PBMT research is infrared neural modulation (INM). INM is centered around the use of pulsed infrared lasers to activate and deactivate neural tissue without causing damage.22 Restoration of neural function, known as neuroprosthese, has been historically hindered by the spatial resolution of electrodes. Since INM is an optical technique, it has much finer spatial resolution, making it more desirable than traditional electrical modulation. It is still in the early stages, and INM is showing great promise as a treatment for a variety of neural impairments. Currently, this technology is being spearheaded by Anita Mahadevan-Jansen, E. Duco Jansen, and the team at the Vanderbilt University’s Biophotonics Center, where they are conducting both fundamental research and clinical trials.
Final thoughts
Many of the technologies discussed are still in the research phases, but it is clear that the future of photonics in healthcare will be centered around nondestructive and minimally invasive laser techniques. While people may not realize it just yet, the medical community is rapidly moving towards a day when, just like in Star Trek and other science fiction, a doctor can quickly diagnose patients using one light source and then treat them using another. To quote Brian Pryor, CEO of LiteCure LLC and Companion Animal Health, “the story of light in medicine is still being written.”
REFERENCES
1. V. Ilardi, “Renaissance vision from spectacles to telescopes,” American Philosophical Society, 259 (2007).
2. A. J. Wollman et al., Open Biol., 5, 4, 150019 (2015).
3. National Research Council, “Optics and Photonics: Essential Technologies for our Nation,” National Academies Press (2013).
4. P. E. McGuff et al., Can. Med. Assoc. J., 91, 21, 1089 (1964).
5. J. A. Salo et al., Annals Surg., 227, 1, 40 (1998).
6. Q. Peng et al., Rep. Prog. Phys., 71, 5, 056701 (2008).
7. J. D. Horsnell et al., The Surgeon, 10, 3, 123–127 (2012).
8. J. Horsnell et al., Analyst, 135, 12, 3042–3047 (2010).
9. R. Thomas et al., Spectroscopy, 28, 9, 2–8 (2013).
10. R. Chimenti et al., Am. Pharm. Rev., 18, 1, 10–14 (2015).
11. P. Matousek et al., Appl. Spectrosc., 59, 4, 393–400 (2005).
12. G. Feng et al., J. Biophoton., 10, 8, 990–996 (2017).
13. N. Stone et al., Analyst, 132, 9, 899–905 (2007).
14. P. Matousek and N. Stone, J. Biophoton., 6, 1, 7–19 (2013).
15. F. Nicolson et al., Chem. Sci., 9, 15, 3788–3792 (2018).
16. J. F. Ennever et al., J. Pediatr., 103, 2, 295–299 (1983).
17. V. M. Holanda et al., Arquivos Brasileiros de Neurocirurgia: Brazilian Neurosurgery, 37, 4, 317–325 (2018).
18. L. F. de Freitas and M. R. Hamblin, IEEE J. Sel. Top. Quantum Electron., 22, 3, 348–364 (2016).
19. C. Dompe et al., J. Clin. Med., 9, 6, 1724 (2020).
20. P. Arany, Laser Focus World, 55, 8, 22–24 (2019).
21. M. R. Hamblin and T. N. Demidova, Proc. SPIE, 6140, 614001 (Feb. 2006).
22. C. P. Richter et al., Laser Photon. Rev., 5, 1, 68–80 (2011).
About the Author
Robert V. Chimenti
Director, RVC Photonics LLC
Robert V. Chimenti is the Director of RVC Photonics LLC (Pitman, NJ), as well as a Visiting Assistant Professor in the Department of Physics and Astronomy at Rowan University (Glassboro, NJ). He has earned undergraduate degrees in physics, photonics, and business administration, as well as an M.S. in Electro-Optics from the University of Dayton. Over a nearly 20-year career in optics and photonics, he has primarily focused on the development of new laser and spectroscopy applications, with a heavy emphasis on vibrational spectroscopy. He is also very heavily involved in the Federation of Analytical Chemistry and Spectroscopy Societies (FACSS), where he has served for several years as the Workshops Chair for the annual SciX conference and will be taking over as General Chair for the 2021 SciX conference.