Tomasz Tkaczyk
Cassical bench-top microscopes are sophisticated tools based on a universal platform to meet diverse needs without significant reconfiguration. They are ideal for research work, but less so for many emerging applications including disease screening.
Detection of malaria or tuberculosis, for example, requires high-resolution images (from several to several hundred), which makes specimen evaluation lengthy and tedious. In addition, the need for highly trained operators limits the use of standard microscopy and image cytometry in poor and underdeveloped regions.
In fields such as cancer diagnostics, endomicroscopy can enable high-resolution in vivo imaging, increasing the screened tissue volume and providing guidance for acquisition of biopsy samples for subsequent histopathology. Endomicroscopy can also enable in situ tumor margin detection, assessment of targeted therapies, and evaluation of drugs during development.
The ideal platform for such applications would be an easy-to-use, integrated, miniature system.
Give and take for fabrication
While mass production of electronic components has been available for many years, optics fabrication has only recently caught up in this respect. Advances in manufacturing, light sources, sensors, and actuators have lately opened new avenues toward building integrated optoelectromechanical devices that are more robust, compact, economical, and energy efficient than their large-scale counterparts. For example, miniature digital microscopes using small-pixel CMOS/CCD detectors can work at low magnification to deliver high-resolution, diffraction-limited images.
The concept of a miniature digital microscope is compelling. But numerous conditions must be met to enable cost-effectiveness without sacrificing image quality. Some applications–such as endoscopy and integrated microfluidic imaging–also impose strict restrictions on physical dimensions.
In practice, the three parameters of performance, size, and cost are strongly interrelated. For example, high-performance systems require components with tight manufacturing and assembly tolerances, both of which can increase the overall system cost. Small overall dimensions are difficult to achieve at low cost in combination with large field-of-view (FOV) and high numerical aperture (NA), because NA and FOV are in principle inversely proportional for fixed system dimensions. It is not always practical from an application standpoint to reduce an objective’s size to extremely small diameters (for example, below 0.5 mm), because in such situations, the system diameter imposes requirements of small FOV and short working distance if a high NA is to be maintained. Endoscopes with fields of view ranging from 50 to 100 µm might not be very useful, even with 0.5 µm resolution. For these extremely small probes, an alternative to the wide-field mode of imaging may require object point by point scanning with imaging on-axis only (entire probe moves in reference to the sample). Examples using point scanning techniques include OCM (optical coherence microscopy) catheters1 in endoscopy, or microfluidic confocal microscopy.2
Despite these challenges, development of imaging systems with small dimensions isn’t entirely an uphill battle. Fabrication of miniature components can benefit from lithography (direct writing or with high-energy-beam-sensitive glass masks) or micromilling, both of which are impractical for large-scale optics. These techniques make it possible to fabricate optical components with arbitrary or aspheric surface figures as easily as spherical surfaces. The capability can simplify optical design and reduce the number of required lenses within a system. Similarly, miniature optomechanical components can be made with DRIE (deep reactive-ion etching) or LIGA (from the German acronym lithographie galvanoformung und abformung, meaning deep x-ray lithography and electrodeposition). The superior accuracy of such techniques enables assembly with high positioning tolerances (on the order of a few microns). This allows implementation of the zero alignment concept,3 which relies on these technologies to produce components that can be assembled in a plug-and-snap fashion without alignment or adjustment. Both optical and optomechanical elements have to accommodate reference features that provide self-alignment during assembly.
A few examples
In recent years several high-resolution miniature microscopes have been demonstrated. Most are built for endomicroscopy,4-12 microfluidics/point of care,13 and telepathology applications.14
Let’s look at a few examples of multielement high-performance miniature microscopes.5-8, 15-17 The samples discussed were developed in collaborations between the University of Arizona (College of Optical Sciences) and Rice University (Department of Bioengineering). While there are other examples,most are proprietary and access to detailed information is limited. 4, 9-10, 13-14
All of the devices described here are multilens optical systems, with outer diameters ranging between 3 and 8 mm, numerical apertures ranging from 0.4 to 1.0 (water immersion), and FOV of 250 microns.3-5 They operate with low transverse magnification (3&time; -4&time;), using small-pixel cameras or optical fibers as detectors.
The primary application for these devices, early cancer detection, requires small system diameters to permit access through standard endoscopes while maintaining adequate FOV and resolution (NA). For other applications such as point-of-care diagnostic or telepathology, the small-diameter requirement can be relaxed. A motivation for development of these microscopes was the wish for cost-effective technology to widen access to high-resolution imaging and routine screening methods.
The 4M Device
While the first development step is usually design and manufacture of the miniature objective, it is possible to extend the objective’s functionality so that a complete digital microscope can be built–rather than a stand-alone optical system. Particularly interesting in this regard is the integrated NA = 0.4 Multi-Modal-Miniature-Microscope (4M device) (see Fig. 1).7, 16 This device is based on a micro optical table (MOT) and consists of an optical system with one glass spherical lens and three aspheric lenses made with grayscale lithography in sol-gel, a beamsplitter or dichroic mirror (depending on imaging mode), electrostatic comb drive with Ronchi grating to provide structured illumination, and a condenser lens. Light is coupled into the system through a multimode fiber from a high-power LED source, with images collected on a miniature high-speed CMOS image sensor.
The high-power front lens and condenser lens were assembled using self-centering mounts, inherently compensating for low centration tolerances in each element. Sol-gel lithographic lenses were made on thin glass substrates and incorporated in the system with kinematic mounts. Sol-gel lenses were tilted to reject stray light from the system while their relatively complex aspheric form compensated for aberrations resulting from these tilts.18 The MOT substrate and optomechanical components were fabricated with nickel-steel alloy using LIGA.
Objectively speaking
Low-cost, efficient fabrication of miniature microscope objectives is key, especially for point-of-care systems for detection of infectious disease or cancer. In one example, a system consisting of five aspheric lens elements incorporates kinematic mounts into the objective parts (see Fig. 2a).6, 8 The integrated objective was built using one spherical glass lens and two plastic aspheres. The glass lens (with refractive index above 1.8) provides high power, while the aspheric components allow correction of aberrations and reduces the total number of lens elements. Optomechanical fixtures were made with LIGA and include microscale self-centering mounts. This objective also incorporates hydraulic lines, which can enable axial translation of the object plane or be used for drug or contrast agent delivery during in vivo imaging (see Fig. 2b). Several stacked layers are combined with precision pins and bonded after assembly. The outside diameter of this system is 3.8 mm; it can easily attach to a coherent fiber bundle by means of a miniature spring latch to enable fiber-optic confocal microscopy.An improved version of this objective uses simpler optomechanics and is built with standard tolerance (±50 µm diameter) hypodermic tubing (see Fig. 2c, left).19 The self-centering mounts compensate for the loose tolerances of both the hypodermic tubing and the inexpensive glass spherical lens. This capability is particularly important because the acceptable decentration error for this NA = 1.0 design is below ±5 µm. These systems usually reach or approach diffraction-limited optical performance.
Disease detection
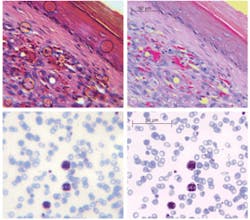
Miniature microscopes can be useful in detection of cancer and infectious diseases (see Fig. 3). Even with a miniature objective designed for imaging at near-infrared wavelengths (810 nm) and having 5 µm field curvature (for thick tissue imaging this parameter is not critical and simplifies designs) it is possible to detect malarial parasites, conventionally identified using 100&time; oil immersion systems. In the images, abundant red blood cells (~8 µm diameter) appear blue/gray, with three larger white blood cells demonstrating uptake of the violet stain. The much smaller (less than 1 µm) dark violet dots within red blood cells are malaria parasites.Designed as simple systems or as stand-alone chip-based instruments, miniature microscopes can range in cost from $50 to $200. Despite considerable advances, though, several challenges remain unsolved. We need, for instance, new optical materials for cost-effective injection molding and lithography, to improve correction of chromatic aberrations. Fabrication technologies also must be advanced. High-refractive-index polymers may further simplify optical designs by reducing the number of components in an objective system. Other important issues include assembly (the step that currently contributes the most to overall cost), and the interconnection of optics with electronics and actuators. The biggest single challenge, however, is implementation of mass production, to bring these sophisticated miniature optical systems to everyday use.
REFERENCES
- G.J. Tearney et al., Lasers and Electro-Optics, 1996, CLEO apos 96, p. 57 (June 1996).
- X. Heng et al., Lab Chip 6, p. 1274 (2006)
- M.R. Descour et al., IEEE J. Quant. Elect. 38(2) p. 122 (2002).
- A.R. Rouse et al., Appl. Optics 43(31) p. 5783 (2004).
- C. Liang et al., Optics Exp. 9, p. 821 (2001).
- M.D. Chidley et al., Appl. Opt. 45, p. 2545 (2006).
- J.D. Rogers et al., J. Biomedical Optics 13 (5), p. 054020.1 (2008).
- R.T. Kester et al., Optics Exp. 15, p. 2409 (2007).
- P. Delaney et al., Proc. SPIE 6432, p. 64320G (2007).
- F. Jean et al., Optics Exp. 15, p. 4008 (2007).
- C.J. Engelbrecht et al., Optics Exp. 16, p. 5556 (2008).
- M.T. Myaing et al., Optics Lett. 31(8) (2006).
- Y. Gambin et al., Appl. Phys. Lett. 88 (17) Art. #174102, ISSN 0003-6951 (2006).
- A. Olszak et al., OE Magazine (May 2005).
- M.D. Chidley, et al., Proc. SPIE 6082, p. 50 (2006).
- T. Tkaczyk et al., Proc. SPIE 5959, p. 138, Medical Imaging; Warsaw September (2005).
- J.D. Rogers et al., Optics Exp. 12, p. 1294 (2004).
- J. Rogers et al., Optics Lett. 31, p. 504 (2006).
- R.Kester et al., Frontiers in Optics, OSA Annual Meeting, Rochester, NY (2008).
TOMASZ TKACZYK is assistant professor of bioengineering and assistant professor of electrical and computer engineering at Rice University (Houston, TX). Contact him at [email protected].