Natural vanadium-based metamaterial fools IR thermal cameras
Cambridge, MA--A group at the Harvard School of Engineering and Applied Sciences (SEAS) led by Federico Capasso has created a vanadium dioxide (VO2) thin-film coating that intrinsically conceals its own temperature (and what's under it) from thermal IR cameras.1 When heated, the 150-nm-thick film develops an anomalous thermal-emittance profile; the effect is based on the material's transition from an insulator to a metal as it warms.
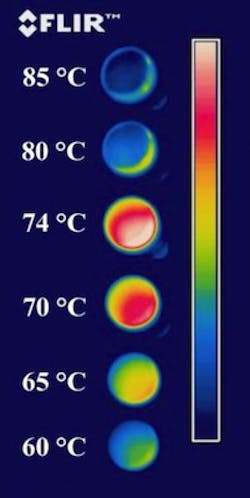
The researchers placed a coated sample on a hot plate and watched it through an IR camera as the temperature rose. Initially, it behaved conventionally, giving off more IR light as the sample was heated (at 60 °C it appeared blue-green to the camera; at 70 °C it was red and yellow; at 74 °C it turned a deep red; see figure). But then, as it warmed further, the apparent temperature plummeted (at 80 °C it looked blue, as if it could be 60°C, and at 85 °C it looked even colder). The effect was reversible and repeatable many times over.
The team discovered that nanoscale structures appearing naturally in the transition region of VO2 can be used to provide tunability. The researchers call the spontaneously structured material a natural, disordered metamaterial.
Active thermal camouflage
Capasso predicts that with only small adjustments the coating could be used as active thermal camouflage or as a kind of encrypted beacon to allow soldiers to covertly communicate their locations in the field.
Vanadium oxide is an unusual material that undergoes dramatic electronic changes when it reaches a particular temperature. At room temperature, pure vanadium oxide is electrically insulating, but at slightly higher temperatures it transitions to a metallic, electrically conductive state. During that transition, the optical properties also change.
The insulator-metal transition has been recognized in VO2 since 1959; however, it is a difficult material to work with. In bulk crystals, the stress of the transition often causes cracks to develop and can shatter the sample. Recent advances in materials synthesis and characterization -- especially those by coauthor Shriram Ramanathan at Harvard SEAS -- have allowed the creation of extremely pure samples of thin-film VO2.
“By introducing impurities or defects in a controlled way via processes known as doping, modifying, or straining the material, it is possible to create a wide range of interesting, important, and predictable behaviors,” says lead author Mikhail Kats, a graduate student in Capasso's group at Harvard SEAS.
By doping VO2 with tungsten, for example, the transition temperature can be brought down to room temperature and the range of temperatures over which the strange thermal radiation effect occurs widened. The researchers say a vehicle coated in VO2 tiles could potentially mimic its environment like a chameleon, appearing invisible to an IR camera with only very slight adjustments to the tiles’ actual temperature, a far more efficient system than conventional approaches.
Covert beacon
Tuned differently, the material could become a component of a covert beacon, displaying a particular thermal signature on cue to an IR surveillance camera. Capasso’s team suggests that the material could be engineered to operate at specific wavelengths, enabling simultaneous use by many individually identifiable soldiers. And, because thermal radiation carries heat, the researchers believe a similar effect could be employed to deliberately speed up or slow down the cooling of structures ranging from houses to satellites.
REFERENCE:
1. Mikhail A. Kats et al., Phys. Rev. X 3, 041004 (2013); doi: 10.1103/PhysRevX.3.041004

John Wallace | Senior Technical Editor (1998-2022)
John Wallace was with Laser Focus World for nearly 25 years, retiring in late June 2022. He obtained a bachelor's degree in mechanical engineering and physics at Rutgers University and a master's in optical engineering at the University of Rochester. Before becoming an editor, John worked as an engineer at RCA, Exxon, Eastman Kodak, and GCA Corporation.