Photonics Applied: Light-Based Energy Production: Transitioning to a sustainable light-based energy future
IBRAHIM DINCER
During the Paris Climate Change Conference in December 2016, it was declared that renewable energy options will be the key in fulfilling carbon-dioxide (CO2) reduction targets and hence limiting the global temperature to no more than a 2°C increase. In another internationally important event, the Davos Economic Forum in January 2017, the chief executives from 13 global companies signed an agreement to establish the Hydrogen Council and stated that it is not possible to meet the energetic and environmental targets without hydrogen production. The need for clean and sustainable methods for producing hydrogen is clearly a primary motivation behind many international initiatives.
Clean energy
At the University of Ontario Institute of Technology’s (UOIT) Clean Energy Research Laboratory (CERL), we are strongly committed to playing a significant role in achieving a carbon-free economy and society through research, innovation, and commercialization of clean hydrogen-production methods.
Our group started its first-ever large-scale project on nuclear-based hydrogen production through the thermochemical copper-chlorine cycle—a key achievement that became the heart of our research program and a starting point for CERL’s quest for sustainable hydrogen production. Since then, CERL has been actively attracting projects and developing technologies in a wide range of clean-energy disciplines.
Excellence in training highly qualified personnel and numerous publications support CERL areas of research, innovation, and technology development, including hydrogen production, fuel cells, heat engines, solar energy, wind energy, geothermal energy, biomass and biofuels, advanced power generation, nuclear energy and non-electric applications, heat pumps, energy conversion and management, environmental impact assessment, and micro- and nanotechnologies.
While solar-driven hydrogen production can be part of the clean-energy solution, ammonia can also be considered as an energy candidate because of its higher volumetric energy density, high capacity for hydrogen storage (17.6 wt.%, based on its molecular structure), and its ability to be easily stored, transported, and even liquified (its 9.2 bar vapor pressure at room temperature is similar to propane). In addition to being used as a medium to store hydrogen, ammonia can also be directly burned in an internal combustion engine without any carbon emission similar to hydrogen.
Several photonic and hybrid photonic/chemical methods are available to produce hydrogen and ammonia. Our team at CERL compares photocatalysts, photocatalytic/electrochemical processes, and solar-cell-driven electrolyzers to better understand the lowest-energy consumption options that will fuel our light-based energy future.
Light-based systems at CERL
At CERL, we focus on theoretical and experimental investigation of photovoltaic (PV), photolysis, photocatalysis, and photoelectrochemical processes under different operating conditions—both in the laboratory and on prototype systems.
Our solar-driven hydrogen production system studies started with low-temperature water electrolysis setups that were driven by PV electricity. Water electrolysis is commonly used for hydrogen production at a commercial scale. Theoretically, water splitting requires a 1.23 V potential difference in an acid medium. However, the true cell voltage is higher—around 1.8 to 2.0 V. A portion or even all of the required electric potential can be provided by PV cell arrays. Both PV and geothermal systems have been developed at CERL for hydrogen production through low-temperature electrolysis.1 These systems can also be assisted with photocatalysts in hybrid reactors.
The solar energy is absorbed by one or both of the photoelectrodes. Semiconductor-based materials in a photoelectrochemical cell (PEC) work using the same principles as PVs and can convert solar energy to an energy carrier such as hydrogen (H2) or with further steps, ammonia, through light-stimulation processes of photo-reduction and photo-oxidation: Therefore, in an electrolyzer cell, electrodes can be either replaced or coated with a semiconductor material to better use solar energy.
Experimental investigations of hybrid photocatalytic water-splitting reactors over an expanded range of the solar spectrum have been carried out at CERL using cadmium sulfide (CdS), zinc sulfide (ZnS), copper oxide (Cu2O), and titanium dioxide (TiO2) catalysts for hydrogen and oxygen production.2-4
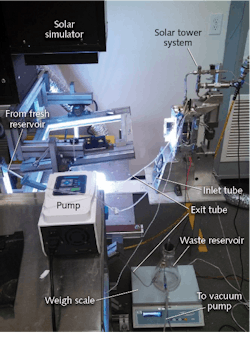
Using both ZnS and CdS nanoparticulate photocatalysts, an integrated solar tower for photocatalytic hydrogen and power production has been demonstrated at CERL (see Fig. 1).5 A heliostat field is arranged to concentrate and collimate radiation towards the opening of the cavity receiver. Solar simulator irradiance is measured by a pyranometer. A heliostat field is located under the solar simulator that consists of 3 cm2 silver mirrors while the receiver has one cavity to allow irradiance inside.
This solar-tower reactor design involves the entrapment of photons within the cavity receiver, thus enhancing the capacity. Besides the continuous H2 production capacity of the system, the hybrid system also converts the by-products of H2 production into useful industrial commodities such as chlorine (Cl2) and sodium hydroxide (NaOH), which are desired by various industries. The system has an annual production capacity of 2.8 kg H2 per square meter of a heliostat with a production cost of $1/kg of H2.
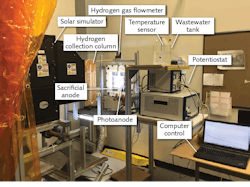
Another PV-assisted hybrid system built at CERL for photoelectrochemical H2 production uses more of the solar spectrum through the combination of photocatalysis and PV processes. Moreover, the system eliminates the electron donor requirement of photocatalysis (Cu2O) by using photoelectrodes that have reduced the risk of potentially harmful pollutant emissions. Comparative studies have been completed to assess the efficacy of different photoactive and coating materials for optimal H2 production.6, 7
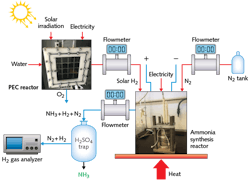
A similar photoelectrochemical (PEC) reactor built by electrodeposition of the photosensitive semiconductor Cu2O on the photocathode instead enables conversion of hydrogen directly into ammonia at atmospheric pressure and low temperature (see Fig. 2). Here, PEC H2 generation is accomplished by a PEC cell with a membrane electrode assembly (MEA), PV array, concentrated light source, and optical tools such as Fresnel lenses and spectrum-splitting mirrors.After Fresnel lens concentration of the solar spectrum, beamsplitting mirrors direct the higher wavelengths of the solar spectrum to the PV cells, while the lower wavelengths of the spectrum are directed to the PEC cell. The electrochemical ammonia synthesis reactor is built using a molten salt electrolyte, nickel electrodes, and iron-oxide catalyst. Synthesis of ammonia succeeds through hydrogen and nitrogen feed gases introduced at temperatures above 180°C and at ambient pressures. The photoelectrochemically produced H2 then reacts with nitrogen in the electrochemical reactor to produce clean ammonia.
Unlike conventional water electrolysis, iron oxidization occurs on the anode side of the reactor. The Fenton reagent is formed inside the reactor by the sacrificial stainless steel anode. Normally for water electrolysis, the theoretical energy for the electrochemical reaction is 237.1 kJ mol-1. Since the anodic reaction of the system is replaced by the Fenton-like processes, the required chemical energy is 163 kJ mol-1 less than conventional electrolysis, meaning that the system consumes less energy while producing H2 and in addition, purifies the water. This PEC reactor can degrade all chemical oxygen demand of the wastewater within 24 hours.
Future directions
At CERL, new materials with high photoactivity in the visible range are seen as essential for the development of commercially viable light-based energy systems. Of particular interests are metal organic framework (MOF) compounds, mesoporous materials, and dye-sensitized and thin-film solar cells. Future direction of light-based energy systems research at CERL include fourth-generation PV, multi-quantum-well structures, quantum dots, nanowire organic cells, thermophotonic conversion, polycrystalline thin films, molecular organic devices, and transparent PV.
Clean production of hydrogen, power, heat, and industrial chemicals, and clean services (such as cleaning, purifying, drying ,and wastewater treatment) is a priority when planning future research activities at CERL. After demonstrating significant achievements in producing hydrogen, ammonia, electricity, and heat in combination with wastewater treatment, we are now moving into a new domain that explores how photonics can be used to produce renewable fuels such as methanol and ethanol.
REFERENCES
1. I. Dincer and G. F. Naterer, Int. J. Hydrog. Energy, 39, 20592–20613 (2014).
2. E. Baniasadi et al., Appl. Catal. A, 455, 25–31 (2013).
3. Y. Bicer, “Investigation of novel ammonia production options using photoelectrochemical hydrogen,” Ph.D. dissertation supervised by I. Dincer, UOIT (2017).
4. G. Chehade et al., “Experimental investigation and analysis of a new photochemical reactor for hydrogen production,” Int. J. Hydrog. Energy (in press; 2018).
5. R. Shamim, “Experimental investigation and analysis of a new light-based hydrogen production system,” MASc. dissertation supervised by I. Dincer, UOIT (2013).
6. C. Acar and I. Dincer, Int. J. Hydrog. Energy, 43, 10249–10257 (2018).
7. C. Acar and I. Dincer, Int. J. Hydrog. Energy, 41, 7950–7959 (2016).
Ibrahim Dincer is editor-in-chief of the International Journal of Energy Research and the International Journal of Global Warming, and professor of mechanical engineering at the University of Ontario Institute of Technology (UOIT), Oshawa, ON, Canada; e-mail: [email protected]; www.engineering.uoit.ca/people/amme/faculty/ibrahim.dincer.php.