Laser developments support advanced neuroscience techniques
Neuroscience researchers are slowly but surely unravelling the mysteries of how the brain works, thanks in large part to studies on the mouse brain as the model for the larger mammalian (i.e., human) brain. A lot of this research involves mapping how neural networks are connected and function, which requires analysis methods with high (single neuron) resolution in three dimensions. Multiphoton scanning microscopes equipped with ultrafast (femtosecond) lasers are key enabling tools that naturally provide this 3D resolution, where the point scanning provides x-y resolution (image plane) and the nonlinear laser excitation takes place only at the tight focus scanned along the z-axis.
Two-photon fluorescence excitation is well established as a tool for neuroscience. In the pursuit of deeper and more selective functional imaging, two other methods have attracted considerable interest: three-photon (3P) fluorescence microscopy and two-photon (2P) optogenetic photostimulation. 3P microscopy is increasingly used as a technique to image deeper into the mouse brain, even beyond the 1-mm-thick cortex. 2P photostimulation of opsin membrane proteins is enabling researchers to activate or silence specific sets of neurons with single neuron resolution. Researchers are also starting to combine the methods to perform all-optical physiology studies, in which a laser stimulates neurons to fire, and a second laser fluorescently maps activity in nearby neurons via changes in their Ca2+ levels that cause changes in the fluorescence intensity of calcium indicators.
These three frontline techniques—two-photon fluorescence excitation, 3P fluorescence excitation, and 2P photostimulation—all have specific and quite different laser requirements in terms of pulse energy, wavelength, repetition rate, etc. Let’s take a look at the latest laser developments that streamline how these beam properties are generated, simplifying their use for the broadest possible user base.
Three-photon imaging
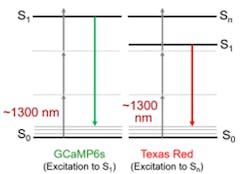
3P microscopy uses laser light at 3X the single-photon absorption wavelength of the target fluorophore—resulting in several advantages for deep imaging (see Fig. 1). For common fluorophores such as green fluorescent protein (GFP) and the genetically expressed calcium indicator (GCaMP), this translates to a laser wavelength of ~1300 nm. The scattering and absorption characteristics of most tissues mean there is a window of high penetration depth at this wavelength. So, 3P microscopy naturally offers greater imaging depth than 2P microscopy. Just as important, the use of 3P excitation can deliver clean images with virtually no out-of-focus fluorescence background (see Fig. 2).But 3P excitation has a third-order dependence on the peak laser power. This means 3P microscopy requires a pulse energy on the sample of 100 to 200 nJ with a short (50–60 fs) pulse width to reach the requisite peak power and focused intensity. 3P microscopy also requires pulse repetition rates of 1 to 2 MHz as a tradeoff between imaging speed and sample viability. Unfortunately, it is challenging to design an easy-to-use laser source that satisfies these requirements. For instance, this pulse energy is 500–1000X higher than the typical energy on the sample used in 2P microscopy and a 2–3X shorter pulse width is required. For these reasons, researchers have used ytterbium ultrafast amplifiers to pump a standalone optical parametric amplifier (OPA) that produces the 1300 nm output.
Even with this type of OPA source at a few megahertz, the imaging speed is still sometimes limiting as experiments target a higher number of neurons simultaneously (i.e., a larger imaging volume). A way around this limitation was addressed in the laboratory of Professor Na Ji (University of California, Berkeley) using the laser/OPA combination. This group also used a home-built pulse compressor to minimize the group delay dispersion (GDD) at the sample plane. Their key innovation, however, was the use of a so-called Bessel focus to create a long focal waist along the z-axis in the sample, which enables a thicker image slice to be recorded within a single frame. Ji notes that an additional benefit of the extended z-axis focal depth is increased immunity to any slight movements of the sample along the z-axis. Excitation with Bessel focus also benefits from three-photon excitation, which suppresses fluorescence generation by its side rings.
In another recent study of 3P imaging at Cornell University, researchers in the laboratory of Professor Chris Xu also used this combination of ytterbium laser and OPA at 1300 nm to demonstrate that this wavelength can excite a broader range of fluorophores than the ones based on GFP. In particular, they showed that this wavelength could also excite many orange and red probes with enough intensity for imaging (see Fig. 1). They then separated fluorescence from the two probe types by using spectral separation in the detection channel.
Another early adopter of 3P imaging is the laboratory of Jack Waters at the Allen Institute (Seattle, USA). An earlier article published in Laser Focus World (February 2000) described their use of the ytterbium amplifier/OPA combination. The mouse cortex has a thickness of about 1 mm, or 1000 µm (see Fig. 3), so as neuroscience mapping studies proceed beyond the cortex, Waters states that it will be critical to use techniques with penetration depths greater than this.Two-photon photostimulation
Optogenetics is a method to activate or silence neurons with light. Specifically, protein complexes called opsins act as ion gates and pumps located in the neuron outer membrane. These open and close when irradiated with light of the appropriate wavelength. Optogenetics provides a noninvasive stimulation technique that offers much higher spatial resolution and flexibility than other behavioral stimulation methods.
Initially, optogenetics used blue light to match the absorption of early opsins. Now, two-photon photostimulation within the 1000–1050 nm window is gaining interest because it offers the same three-dimensional discrimination as multiphoton imaging. However, much higher power is required to activate neurons compared to 2P-excited fluorescence. Moreover, 2P photostimulation experiments typically use spatial light modulators (SLMs) to create diffracted light patterns that then target many neurons simultaneously. This subdivision of the light power and the limited (e.g., 10%) optical efficiency it entails serve to further increase the demand for power. As a result, researchers typically want a source with a few tens of watts. This is ideally matched by the new generation of ytterbium amplifiers with output at 1035 nm.
One-box source for 3P imaging and 2P photostimulation
To better support these advanced neuroscience studies, both the ytterbium laser and 1300 nm wavelength generation have now been combined as a single package, such as the new Monaco 1300 from Coherent, with an output pulse width of <50 fs, a pulse repetition rate of 1, 2, or 4 MHz, and output power of 1.5 or 2.5 W. This new format is a one-box source of 1300 nm output that meets the required parameters for 3P imaging.
This consolidation effort also allows easier integration of two important features for the microscope community. Total power control (TPC) provides on-the-fly power attenuation and fast optical gating to simplify tasks such as blanking during flyback as part of fast raster scanning. Another important option is a compact pulse compressor (CPC) that allows dispersion precompensation for optimum pulse width at the sample. As noted already, short pulse width at the sample is even more important in 3P than in 2P methods because of its third-order dependence on peak power.
While the final 1300 nm output is optimized specifically for 3P imaging, this new laser source can be switched to access the output of the ytterbium pump laser by bypassing the 1300 nm module, providing 60 W of output at 1035 nm, ideal for 2P photostimulation. The user can adjust the pulse repetition rate of this output up to the full 50 MHz native speed of the ytterbium laser.
The end result is a one-box source for both 3P imaging and 2P photostimulation, which reduces the cost and the complexity of performing advanced neuroscience experiments.
2P imaging is still a neuroscience workhorse
While 3P imaging is an important new technique that enables uniquely deep imaging, it is by no means a panacea for all neuroscience imaging experiments. For imaging nearer the surface of tissues, 2P imaging is still preferred because it provides much faster images than 3P—for example, via resonant scanning. It is usually the preferred method for full optical physiology experiments using light for both triggering and interrogating neurons.
In terms of laser performance, 2P imaging needs just a few watts of laser output at a shorter wavelength, e.g., at 920 nm, and repetition rates of 50 to 100 MHz. There is still no single source (yet) that can provide the full complement of all three of these different laser outputs for the neuroscience lab. Specifically:
The 920 nm output, however, can be obtained from one of the compact next-generation single-wavelength lasers introduced by the laser industry during the last couple of years. These femtosecond lasers were designed from the ground up to deliver lower cost, reduced complexity, and an ultracompact footprint for both OEM integrators and end users. One example is the Axon series from Coherent. While compact and economical, these lasers also offer optional high-performance integrated features including TPC and GDD precompensation.
Many important neuroscience studies are making extensive use of multiphoton excitation—for both imaging and optogenetic photostimulation. The laser industry is supporting this research with laser sources that provide optimized output for the three major multiphoton techniques used by neuroscience today. This includes a single source to support two of the techniques—3P imaging and 2P photostimulation—and a compact, cost-effective package for the third important technique, 2P imaging. The combination of these light sources addresses all the current needs of an advanced multiphoton microscopy core lab for neuroscience studies.
Marco Arrigoni
Marco Arrigoni is vice president of marketing at Light Conversion (Vilnius, Lithuania). He previously served as director of marketing at Coherent (Santa Clara, CA) from 2007 through 2023.
Erin Dlugosz | Staff Product Support Engineer, Coherent
Erin Dlugosz is a staff product support engineer at Coherent (Santa Clara, CA).