Versatility, continued advancement help Raman spectroscopy shine
Since its discovery in 1928 by C. V. Raman, the light-scattering spectroscopy technique bearing his name has evolved into a powerful and adaptable tool perfect for an array of applications.
“It can detect most chemicals without needing to have prior knowledge of what one expects to detect, as is the case for sensors based on chemical coatings, for example, where the coating needs to be designed for a specific target analyte,” says Jérôme Michon, co-founder and CEO of InSpek, a France-based company that develops Raman spectroscopy-based sensors for detecting chemical and biological species for sectors including biotechnology, pharmaceuticals, fine chemicals, and the food and beverage industry.
“Our focus has been on using fiber-coupled chips, instead of objective-coupled, to allow for easy manipulation of the chips,” Michon says, noting the commercial potential of the waveguide-enhanced Raman spectroscopy (WERS), which prompted the creation of InSpek in 2021 (see Fig. 1).
Despite obstacles, WERS and other Raman techniques are promising for many areas of R&D.
“There are two main challenges of Raman, which we've encountered as much as everyone else who uses Raman,” he says. “First is the weakness of the Raman signal—out of 10 million input photons, only one to 10 output photons will yield useful Raman information. This means little tolerance in the design of optical components, and we can't afford to lose any extra photons.”
The second challenge exists in processing raw Raman signals. According to Michon, users are typically looking for information about the chemicals they're measuring, not necessarily a graph of intensity versus wavelength. And extracting the exact chemical info from the raw graph requires lots of post-processing.
The information Raman provides can help gather many parameters about analytes themselves as well as the system they're in. “It's a vibrational method and many factors impact the vibrations of molecules, such as their conformation or their environment (pH, temperature, solvent, etc.),” says Michon. “The main advantage of Raman spectroscopy is its versatility.”
And it’s the versatility allowing Raman to take a larger role in bio research.
Molecular imaging
The molecular composition of tissue can provide key insight into its health, helping to identify and discern healthy tissue from cancerous or otherwise ailing tissue. Most medical imaging methods are not sensitive enough in that way, providing information about only structural differences.
Increasingly, researchers are turning to Raman spectroscopy for a more comprehensive view and understanding. “It huge potential in clinical diagnostics because information can be assessed label-free, without contact, and nondestructively,” says Iwan Schie, a professor at the Leibniz Institute of Photonic Technology (Germany) and member of the medical engineering and biotechnology department at the University of Applied Sciences Jena (Germany). “There have been a number of previous studies, which indicated that it is quite capable to detect and delineate cancer from healthy tissues.”
Implementation of fiber optics in a Raman spectroscopy setup is a viable option to enhance the technique, but imaging acquisition, sample topology, and real-time data analysis remain a challenge. Currently, Raman spectroscopy using a fiber optic probe is performed on individual locations of a sample and cannot be used to acquire images because the measurement position of a probe is not linked with the measurement information.
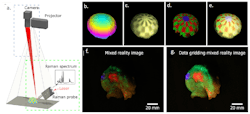
Schie’s team discovered a way to overcome these issues with development of a fiber probe-based handheld imaging system that incorporates real-time data processing and molecular profiling for looking at 3D sample tissue areas (see Fig. 2). The new system combines with augmented and mixed reality to create molecular virtual reality (MVR).“Now we can see directly the molecular information of the measurement location,” he says. “And because many samples also have a topography, meaning different height profiles, we have combined this with a possibility to assess topographic information in our sample.”
In their study, published in Light Science & Applications, the researchers used an imaging platform combining Raman spectra measurements, simultaneous computer vision-based positional tracking with real-time data processing, and real-time formation of images captured via the MVR technique.
Schie notes the MVR images can be observed as augmented chemical reality on a computer screen or directly mapped on the tissue by adding a laser projection system to the process. The team also implemented a photometric stereo measuring system, allowing the molecular information to be mapped on a 3D sample surface.
These developments could prompt clinical translation of real-time Raman-based molecular imaging in the future, becoming a tool for real-time opto-molecular visualization of tissue boundaries for disease diagnostics and surgical resection.
The new technique will “enable the use of Raman imaging in a very different way and to display the information in a more comprehensible way,” Schie says. “It also outlines new possibilities for how we can combine different approaches to create something that is bigger than the individual parts. In future implementation, it could also potentially be adapted to smartphones and used for molecular profiling of everyday goods.”
Virus surveillance, detection
Although the world continues battling the COVID-19 pandemic, scientists are already preparing to combat anything similar if it happens in the future.
A team led by researchers at Pennsylvania State University discovered that Raman spectroscopy could become a tool in such a fight. The team—which also includes researchers from Evan Pugh University, Eberly College of Science, George Washington University, Johns Hopkins University, and the National Institutes of Health—developed a technique using Raman spectroscopy, along with a portable microfluidic device and machine learning, to detect and identify viruses.
According to the study, published in Proceedings of the National Academy of Sciences, a large Raman dataset collected on a variety of viruses enabled the researchers to program machine-learning models capable of highly accurate and sensitive virus identification. Those trained models were integrated with the microfluidic device, ultimately demonstrating the ability to detect viruses in real time. The new method is also label-free and doesn’t target any specific virus; this means it can identify not only known viruses, but also new strains and/or variations. Pairing Raman spectroscopy with machine-learning analysis offers a better understanding of virus structures, as well.
This rapid and label-free virus detection system for virus surveillance could enable public health officials to more closely monitor the evolution of a virus, says study co-author Yin-Ting Yeh, an assistant research professor in the physics department at Eberly College.
Yeh’s method uses Raman to detect unique vibrations in molecules by picking up shifts when a laser light beam induces vibrations. The microfluidic device involved in the system needs only small amounts of body fluid—including virus cultures, saliva, nasal washes, or exhaled breath—to be able to perform lab and medical tests. Such samples could potentially be collected on-site in real time during a virus outbreak.
The device captures viruses by trapping them between groups of aligned carbon nanotubes, developed by Yeh, a materials scientist who studies nanotubes. These “forests” of carbon nanotubes filter out foreign substances and background molecules from the virus’ host or from the surrounding air, ensuring accurate testing.
Yeh’s team captured samples and examined them with a Raman microscope. Several different categories of viruses—human respiratory, avian, and entero-viruses—were studied. The Raman spectra data was used to train a convolutional neural network that identifies viruses. This machine-learning model could then distinguish specific viruses.
The new Raman-involved technique overcomes long-standing challenges posed by existing methods. Traditionally, the gold standard for virus detection has been polymerase chain reaction (PCR) testing, which detects genetic material from a virus or other organism. PCR can detect the presence of a virus at the time of the test, and even fragments of it after an infection has cleared. Of course, the method is very narrow in scope. “You need to know ahead of time what you are detecting. It’s also challenging if the known virus has mutated,” says Yeh. “In that case, you have nothing, you can no longer detect it. And unfortunately, viruses can very easily mutate.”
Label- and labor-free, unlike PCR testing, Yeh’s technique can target highly mutating viruses—such as influenza.
“We’re trying to go beyond viruses, too, to detect and capture the parasites and bacteria inside viruses,” Yeh says, noting this could be particularly useful in areas with limited lab testing resources such as parts of Africa and South America. “We are living with viruses now. There are different viruses circulating in our environment, so we need to keep monitoring them to deal with future outbreaks.”
About the Author
Justine Murphy
Multimedia Director, Digital Infrastructure
Justine Murphy is the multimedia director for Endeavor Business Media's Digital Infrastructure Group. She is a multiple award-winning writer and editor with more 20 years of experience in newspaper publishing as well as public relations, marketing, and communications. For nearly 10 years, she has covered all facets of the optics and photonics industry as an editor, writer, web news anchor, and podcast host for an internationally reaching magazine publishing company. Her work has earned accolades from the New England Press Association as well as the SIIA/Jesse H. Neal Awards. She received a B.A. from the Massachusetts College of Liberal Arts.