Bioimaging advance aids creation of enhanced clinical devices
A new label-free, noninvasive method—coherent anti-Stokes Raman scattering (CARS) microscopy—acquires images of biological samples and the chemical constituents of human tissues and cells quicker and more efficiently than possible with existing techniques. The potential? More efficient clinical devices for disease diagnosis.
A team led by researcher Dario Polli, an associate professor of physics at Politecnico di Milano (Milan, Italy), in conjunction with researchers from Humanitas Research Hospital (Milan, Italy) and the Italian National Research Council’s Institute for Genetic and Biomedical Research and Institute for Photonics and Nanotechnologies, developed the CARS microscope and technique. The technology exploits Raman-based advantages, including label-free modality and chemical specificity, at higher speeds.
Beyond standard methods
Raman spectroscopy, a standard label-free and noninvasive chemical analysis technique, provides vibrational spectra of biological samples—namely cells and tissues—creating a unique signature for identifying their chemical components.
The simplest approach for Raman imaging of biological samples exploits quasi-monochromatic visible or near-infrared laser light to illuminate the specimens. It also measures the spontaneously emitted, inelastically scattered spectra that carry the vibrational information. “However, this technique suffers from very low scattering cross-sections, requiring long acquisition times to the order of ~1 s per pixel, preventing high-speed imaging,” says Polli.
The CARS technique overcomes this limitation and provides higher speeds by several orders of magnitude, thanks to the coherent excitation of the molecules at the focal plane. Polli’s method exploits the interaction between two ultrashort laser pulses—the pump and the Stokes—as well as the biological samples to retrieve information about how molecules vibrate when “tickled” by the laser beams.
Fluorescence microscopy and spontaneous Raman (SR) microscopy are other standard imaging methods used to image biological samples.
Fluorescence microscopy, a fast-imaging technique that offers heightened sensitivity, requires chemically specific fluorescent markers. Adding markers can prompt strong distress in the investigated cells or tissues and potentially interfere with their biological function. Such work benefits much more from SR microscopy, a label-free technique that doesn’t require fluorescent markers.
“SR microscopy allows the user to selectively distinguish many biomolecules in biological tissues,” says Politecnico di Milano doctoral student Federico Vernuccio, citing a drawback: “It is quite slow and does not intrinsically provide 3D sectioning capabilities.”
Overcoming obstacles
As a third-order, nonlinear optical process, CARS overcomes the limitations of fluorescence and SR techniques and enables label-free 3D sectioning without requiring any confocal aperture. It also allows imaging of samples at higher speed than SR microscopy, thanks to the coherent excitation of the molecules at the sample plane.
The CARS system runs at a 2 MHz rate, which is a much lower repetition rate than standard systems. This allows a temporal delay of 0.5 µs between two consecutive pulses, leaving more time in the system for thermal energy dissipation and ultimately reduced photothermal damage.
The ability to generate broadband, red-shifted Stokes pulses that cover the whole fingerprint vibrational region is also advantageous. It uses white-light supercontinuum (WLC) generation in a bulk crystal rather than in photonic-crystal fibers (see Fig. 1). WLC in bulk media is a more compact, robust, simple, and alignment-insensitive technique, yielding a much simpler technical solution, explains Polli.
“WLC exhibits high mutual correlations between the intensities of its spectral components, low pulse-to-pulse fluctuations, and excellent long-term stability, which is comparable to that of the pump laser source itself,” he says. “Plus, given average power at the focus, limited by sample degradation, a low repetition rate entails higher pulse energy and higher peak intensity, which generates a stronger CARS signal thanks to the nonlinear nature of the optical effect.”
Performance is boosted further by its setup (see Fig. 2), which works in a red-shifted spectral region (1035 nm for the pump and 1050–1300 nm for the Stokes beam). The researchers attribute the higher laser intensities on the sample before the onset of photodamage to reduced multiphoton absorption from cell/tissue pigments and DNA.“We use a data-processing pipeline that combines artificial intelligence methods and numerical algorithms, extracting the maximum amount of information from the recorded CARS images,” Polli says. “Our microscope delivers high-quality images at state-of-the-art acquisition speed, with <1 ms pixel dwell time, limited by the spectrometer refresh rate and without compromising sample integrity.”
The CARS technology further provides access to the fingerprint region—a specific portion of the vibrational spectrum of molecules. This region is difficult to detect, says nonlinear optics researcher Giulio Cerullo, a physics professor at the Politecnico di Milano, because it features weak signals, but “it carries the unique signature of each molecule since different compounds produce different patterns of peaks in this spectral region.”
The broadband approach of this technique allows for the collection of information in a single vibrational mode, covering the entire crucial fingerprint region in a single exposure time. To do this, the researchers generated a narrowband pump beam with 10 cm-1 full-width at half-maximum intensity. This accounts for the spectral resolution, according to their study—published in Optics Express.
“These characteristics of the experimental setup allow us to shorten the pixel dwell times down to less than 1 ms to collect CARS spectra, thus boosting the speed of vibrational imaging techniques and enabling high-speed CARS microscopy,” Polli says.
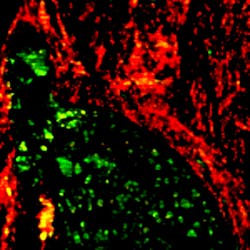
The technique acquires hyperspectral data, as well. This, combined with both the deep learning-based and numerical algorithms, can deliver chemical maps distinguishing the different chemical species in heterogeneous biological samples (see Fig. 3).CRIMSON project
This work is part of the CRIMSON project, a European Commission-funded initiative that seeks to provide a next-generation biophotonics imaging device based on vibrational spectroscopy, with the potential to revolutionize the study of the cellular origin of diseases, allowing for novel approaches toward personalized therapy.
According to Polli, who also serves as the coordinator of CRIMSON, the project focuses on the development of label-free broadband coherent Raman scattering imaging schemes in the fingerprint spectral range with the highest sensitivity and imaging speed.
“In combination with artificial intelligence spectroscopic data analysis, we aim to provide a turnkey instrument for fast cell/tissue classification with unprecedented biochemical sensitivity,” he says.
His research team’s CARS microscopy development and work is among the top milestones reached at the end of CRIMSON’s first year. “Our system may answer urgent and timely biomedical questions, especially in the field of cancer research,” says Polli. “For example, analyzing the interaction of cancer cells with immune cells in head and neck cancers, and characterizing and detecting at an early stage chemotherapy-induced senescent cells.”
Histopathology may also benefit from the advantages provided by the new CARS system since relatively large sample areas must be visualized and characterized to provide accurate diagnosis. “The detection of complex vibrational features over the entire fingerprint region, with high spatial resolution and covering relevant tissue regions, would help to introduce Raman-based spectral histopathology in clinical settings,” he says.

Justine Murphy | Multimedia Director, Digital Infrastructure
Justine Murphy is the multimedia director for Endeavor Business Media's Digital Infrastructure Group. She is a multiple award-winning writer and editor with more 20 years of experience in newspaper publishing as well as public relations, marketing, and communications. For nearly 10 years, she has covered all facets of the optics and photonics industry as an editor, writer, web news anchor, and podcast host for an internationally reaching magazine publishing company. Her work has earned accolades from the New England Press Association as well as the SIIA/Jesse H. Neal Awards. She received a B.A. from the Massachusetts College of Liberal Arts.