Many advances in two-photon microscopy have been made in recent years, including new genetically encoded fluorescent sensors, measurements that multiplex spatial information in time, and microscopes that can view larger or multiple areas simultaneously.
Each generation of ultrafast pulsed lasers enables new developments, most notably colliding-pulse mode-locking (CPM) dye lasers that provided the first generation of ultrafast laser pulses, thanks to Watt Webb—a biophysicist who developed the first two-photon fluorescence microscope in the early 1980s alongside physicists Winfried Denk and James Strickler—to the regenerative chirped-pulse amplification systems Denk used a decade later to push to imaging depths beyond 1 mm with high-power ultrafast pulses, to the current market-leading tunable titanium sapphire (Ti:sapphire) oscillators.
The emergence of next-generation fixed-frequency fiber laser technologies during the last few years is no exception. These advances enable a new class of measurements, including miniaturized microscopes and mobile setups.
Two-photon microscopy advantages
Two-photon imaging is a form of fluorescence imaging. Instead of direct excitation with visible light, it uses two-photon excitation of a fluorescent label with light in the near-infrared (near-IR). While the Abbe diffraction-limited spot size is larger in the near-IR, the two-photon absorption process is quadratic, not linear, in intensity.
The fluorescence excitation and emission are limited to the focal volume instead of a cone of excitation such as in standard fluorescence imaging. This small focal volume provides a much higher depth resolution than the one-photon equivalent and can be scanned across a sample with resonance galvo scanners or acousto-optics. A two-photon microscope can collect an image in 3D by scanning multiple depth slices. And because near-IR penetrates tissue with less scattering than visible light, two-photon microscopy is often paired with genetically encoded sensors for in vivo imaging.
Next-gen applications of fiber laser technologies
The original table-sized apparatus for two-photon microscopy (developed by Webb et al.) has since been reduced to a mere 3 grams. The continuous-wave miniscope was adapted for two-photon depth imaging within a decade of its inception, and a two-photon imaging laser now has a footprint smaller than a large dictionary.
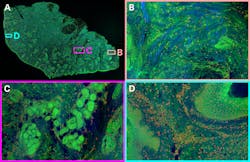
Since 2017, Weijian Zong, a researcher with the Moser group at the Kavli Institute in Norway, has been working on miniaturizing two-photon microscopy. He expanded on a previous two-photon miniscope with the development of the MINI2P—a 3 g head-mounted microscope with a much larger field of view that can image up to 1000 neurons in multiple focal planes in freely moving mice (see Fig. 1).1
Micro-optics play a huge role in this miniaturization, with a MEMS piezo lens quartet for variable focusing and a MEMS scanner for raster scanning. While previous generations of the MINI2P used Ti:sapphire systems for the best quality pulses and best image quality, it limited the mobility of the research setup. For the MINI2P project, Weijian wanted to use his fiber-tethered microscope in different environments, from mazes to cages with climbing walls. So, he built a mobile cart and outfitted it with a TOPTICA FemtoFiber Ultra 920 fiber laser, selected from those available on the market for the best quality sech2 pulses without pulse pedestals or wings.
Fiber coupling the 920 nm output from his laser into his MINI2P microscope, Weijian images genetically encoded GCaMP—a calcium-sensitive fluorescent protein that reveals neural activity—in freely moving mice as they explore their environment. Other setups involve head-fixed mice, in some cases walking around on a styrofoam ball while immersed in a virtual reality environment of computer screens. But such setups limit researchers’ ability to study rodent brains during activities such as climbing or jumping. The MINI2P microscope enables researchers to directly explore these behaviors, providing a new dimension of insight into correlations between behavior and cognition on the cellular level.
Histology on a cart: two-photon microscopy for dermatology
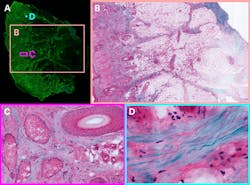
Michael Giacomelli at the University of Rochester is bringing two-photon microscope technology into dermatology offices for same-day screenings. The first thing visible when entering is his soldering station. It’s here that Giacomelli’s group developed the next-generation silicon photomultiplier detectors at the core of the microscope, with about 9X higher signal-to-noise ratio than PMT detectors conventionally used in two-photon microscopy.2
Using this technology, whole-sample images can be collected surprisingly quickly. A sample of cleared tissue, about 1 square centimeter mounted on a microscope slide, can be fully imaged in total to sub-cellular detail in under 30 seconds.3 The researchers don’t need to worry about thermal damage because they’re imaging slices of tissue, not living mice. Brightness can be increased for the highest signal-to-noise, and then to stitch hundreds of two-photon images together to make a centimeter-scale zoomable image (see Fig. 2).Multi-color imaging in vivo
Chris Schaffer and Nozomi Nishimura, researchers at the Meinig School of Bioengineering at Cornell University, developed a hyperspectral multiphoton microscope that uses three laser sources for excitation, at 800, 900, and 1030 nm, instead of the typical one or two. It can detect four channels simultaneously with four different positions of angle-tunable bandpass filters, for fluorescence detection at a total of 16 unique wavelengths. Combined, they collect 48 channels of information per two-photon image. By applying a linear transformation on these channels, based on the known spectra of their fluorescent stains, they demonstrated that they could resolve up to 10 different dyes, and up to eight different tissue types in the same imaging volume.4
One bottleneck of this kind of measurement is the synchronization of the three excitation laser sources and switching between them. With sequential imaging with each laser in vivo, the researchers risked a change in the sample in the time it took to collect an image—a twitch of a muscle or a pulse of a blood vessel—making it difficult to collect and transform these images. Interleaved and optically synchronized laser pulses are an obvious solution.
Advancements in fiber laser technology
TOPTICA’s fiber lasers are one example of advancements made within this realm. They’re built with telecom components, and a mode-locked erbium-doped fiber oscillator generates high-quality, clean laser pulses that are then branched to seed proprietary frequency shift and amplification modules.
Clean pulse technology and 100 fs sech2 pulses provide the highest peak power for a given average power, and best image brightness, for two-photon microscopy applications. And with the same oscillator technology for 780, 920, and 1050 nm laser systems, it is simple to branch up to four lasers seeded by the same oscillator for optically synchronized lasers with a fixed delay between 0 and 12.5 ns, and variable delays up to 500 ps for interleaved pulses. All-fiber propagation, except for the laser head that sits on the optical table, make this a laser system with no need for service contracts or bi-yearly maintenance.
REFERENCES
1. W. Zong et al., Cell, 185, 1240 (2022).
2. V. D. Ching-Roa, E. M. Olson, S. F. Ibrahim, R. Torres, and M. G. Giacomelli, Sci. Rep., 11, 5248 (2021).
3. V. D. Ching-Roa, C. Z. Huang, S. F. Ibrahim, B. R. Smoller, and M. G. Giacomelli, JAMA Dermatol., 158, 1175 (2022).
4. A. J. Bares et al., Optica, 7, 1587 (2020).
Joseph Mastron | Ultrafast Applications Scientist, TOPTICA Photonics
Joseph Mastron, Ph.D., is an ultrafast applications scientist at TOPTICA Photonics (Pittsford, NY).