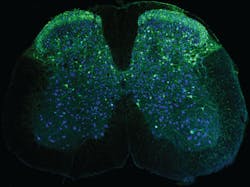
Wearable microscopes help image cellular activity in spinal cords of on-the-move mice
Salk Institute (La Jolla, CA) researchers recently invented wearable microscopes to image and explore previously inaccessible spinal cord regions in mice (see video).
The spinal cord regulates aspects of the body such as breathing and movement. It also plays a vital role in communicating signs of pain by relaying signals between the body and the brain. Conventional imaging technology limits researchers’ understanding of how these processes occur on a cellular level.
Designing tiny microscopes
Commercial microscopes typically offer “either high spatial resolution across a small field of view or low spatial resolution across a large field of view,” says Axel Nimmerjahn, an associate professor and director of Salk’s Waitt Advanced Biophotonics Center. “Achieving high resolution across a large field of view is challenging. And achieving it in a microscope the diameter of your small finger and weighing just a few grams is even more challenging.”
But that’s exactly what the team achieved with the larger of their two microscopes. It can image tissue regions that are 12 to 13X larger than previously possible, resolving individual cells and cell processes across a field of view large enough to cover multiple spinal segments.
This allows simultaneous recordings from up to several thousand cells, Nimmerjahn says. The team can now see cells processing sensory information from the leg’s sciatic nerve; areas affected by sciatica are distributed over multiple spinal segments, and the researchers can now actively image it simultaneously and in real time.
“This enables us to understand better how sensory information, including pain signals, gets processed by the spinal cord,” Nimmerjahn says. “It isn't simply a link/information highway between the brain and peripheral organs, but instead it actively processes information.”
The smaller of the two newly developed wearable microscopes, combined with tissue-compatible and implantable micro-optics (e.g., a microprism), allows the researchers to simultaneously record cells located at different spinal cord depths—areas previously inaccessible. Now, they can better understand how sensory inputs are converted into motor outputs under normal and disease conditions.
When someone touches a hot stove, the sensory information is quickly transformed into a reflexive motor response that helps avoid burn injuries. This sensorimotor information flow can be disrupted or dysregulated in spinal cord injury and diseases.
The team’s work provides insight into the neural basis of sensations and movement in healthy and disease areas and clearer understanding of things like chronic pain, amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS).
“Knowing how the spinal cord usually processes information, and how injury and disease disrupts it, can help us devise treatment strategies to restore sensorimotor function,” Nimmerjahn says.
Multicolor imaging
Previous wearable microscopes had limited ability to image in multiple colors, as well. Multicolor imaging helps uncover cell-cell interactions, which can be highly complex in disease settings. Earlier microscopes also limit working distance, making it difficult to study cells deep inside tissues. The smaller of the two microscopes overcome these and other such challenges.“It allows us to record cells located at different depths within the spinal cord simultaneously, at video-rate speeds and in multiple colors,” Nimmerjahn says.
By viewing sensation- and movement-related nerve activity, the new wearable microscopes are allowing researchers to gather new information about the central nervous system, too, specifically astrocytes—non-neuronal glial cells involved in pain processing.
The team is also investigating how different inflammatory and neuropathic pain conditions and neurodegenerative diseases, such as MS and ALS, alter the normal activity of neuronal and non-neuronal cell types, and how treatments help control these abnormal dynamics.
Mice on the move
Obtaining cellular activity measurements from the spinal cords of mice on the move has been extremely challenging.
During locomotion, the spinal cord moves back and forth within the spine by an amount many times the size of individual cells. It also shows nonlinear distortions (i.e., different parts of the spinal cord move by different amounts), which has prevented cellular measurements via traditional approaches such as electrophysiology.
“In contrast, imaging allows us to track the cells even during vigorous movements reliably,” Nimmerjahn says. “Because of previous technical limitations, the spinal cord’s inner workings are poorly understood. Our microscopes enable us to peer into this ‘black box’ and shed light on cellular and molecular signaling in health, disease, and treatment. Our wearable microscopes have powerful capabilities and provide a glimpse of what is now possible.”The team’s goal now is to identify better treatment strategies for pathologies such as MS and ALS. Their current research involves mouse models, but Nimmerjahn says the team plans to work with physicians and pharmaceutical companies to conduct clinical trials in humans.

Justine Murphy | Multimedia Director, Digital Infrastructure
Justine Murphy is the multimedia director for Endeavor Business Media's Digital Infrastructure Group. She is a multiple award-winning writer and editor with more 20 years of experience in newspaper publishing as well as public relations, marketing, and communications. For nearly 10 years, she has covered all facets of the optics and photonics industry as an editor, writer, web news anchor, and podcast host for an internationally reaching magazine publishing company. Her work has earned accolades from the New England Press Association as well as the SIIA/Jesse H. Neal Awards. She received a B.A. from the Massachusetts College of Liberal Arts.