Crystal ribcage opens black box of a functioning lung to optical imaging
Lungs are perpetually in motion and continuously exposed to pathogens, pollutants, and fatal pathologies such as primary and metastatic cancers. Visualizing the healthy or diseased lung at cellular scales while it functions has been an unmet need. Noninvasive computed tomography (CT) and magnetic resonance imaging (MRI) technologies lack spatial resolution, while the existing real-time intravital microscopy compromises the local ventilation of the lung.
Recognizing this, we developed a transparent “crystal” ribcage that enables the first real-time, high-resolution optical imaging of the functioning lung from whole organ down to single-cell scales. Our platform is being used to probe key pathologies such as cancer, metastasis, pneumonia, fibrosis, and emphysema over the entire surface of functioning lungs.
Why is optical microscopy important for lungs?
The lung is the site of many fatal pathologies, such as primary and metastatic cancers, respiratory infections, as well as both obstructive and restrictive diseases. All these pathologies impact the chemistry, biology, and mechanics of lung structure and function down to the cellular level. Our lungs are continuously in motion and enclosed by the ribcage, which makes studying the functioning lung in real time a challenge.
Existing clinical imaging modalities such as MRI and CT do not have the spatial resolution to capture single cell events, and histological analyses are just a snapshot in time that lack the dynamic interaction between cells as the lung is breathing.
Recent advances in intravital microscopy allowed researchers to probe circulatory dynamics within a small region of the lung at cellular scales by adhering the lung surface to a coverslip via vacuum or glue. This led to major discoveries in lung biology and immunity, but compromised the local respiratory function of the lung.
We developed a transparent crystal ribcage, which enables high-resolution optical imaging of nearly the entire surface of a functioning mouse lung in real time (see Fig. 1). Using the crystal ribcage, we studied the earliest stages of disease development and how it remodels both the structure and function of the lung at multiple scales down to the single cell.
Our platform combines the major benefits of in vivo systems (preserved cellular diversity and complex lung architecture) with “organ-on-chip” capabilities (optical imaging and high controllability of microphysiology), which offers significant opportunities to probe the causal relationships underlying progression of pulmonary diseases—and effectively opens the black box of the lung in both health and disease.
Design of the crystal ribcage and its applications
Our crystal ribcage is a transparent, biocompatible platform designed to provide a physiological environment to enable truly multiscale optical imaging over the entire surface of the functioning lung ex vivo (see Fig. 2). We engineered the geometry and surface conditions to mimic the native ribcage, including making them age- and strain-specific. And we developed a rigid polystyrene crystal ribcage to support “quiet” diaphragmatic breathing through both positive-pressure (mechanical) and negative pressure (spontaneous) ventilation, and a flexible poly-dimethyl-siloxane (PDMS) ribcage to mimic macro-deformations of the ribcage that occur during exercise, coughing, or sighing.
Both materials are biocompatible, ubiquitous in biological research, and free of autofluorescence—making it conducive for laser scanning confocal, spinning disk confocal, and multiphoton microscopy, and optical coherence tomography (OCT) using high-powered objectives (see Fig. 3). The crystal ribcage platform is designed to be modular and microscope agnostic, so it can be used with any top-down or bottom-up configured microscope without compromising imageable area over the lung surface from whole lobe down to single alveoli. We leveraged these capabilities of our platform to probe anatomically localized pulmonary diseases such as fibrosis, lobar pneumonia, and lung cancer metastases.
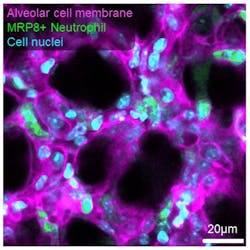
The platform preserves the three-dimensional architecture, air-liquid interface, cellular diversity, and respiratory-circulatory functions of the lung to probe disease progression in mouse models of primary and metastatic lung cancer, pneumonia, pulmonary fibrosis, emphysema, and acute lung injury. In cancer, we observed single cancer cell compression during ventilation and differential remodeling in nodular vs. infiltrative tumors at the single alveolus level, including disrupted circulatory dynamics by single cell clusters up to established nodules (see Fig. 4).
In pneumonia, we observed neutrophil invasion compromising alveolar ventilation and capillary integrity, which potentially affect oxygen and nutrient delivery. In acute lung injury, we observed real-time mechanoresponsiveness of neutrophils, with migration speeds correlated to vascular pressure.
Lastly, we demonstrated respiration-circulation coupling at the capillary level and found that it was eliminated in acute injury. The controllability of lung physiology in the crystal ribcage offers significant opportunities for gaining a mechanistic understanding of pulmonary pathogenesis.
Future directions
The crystal ribcage is a versatile tool for studying various lung diseases with parenchymal presentations, as traditional microscopy is often too slow to capture the full range of physiological lung dynamics. To further enhance our platform, we aim to combine it with next-generation ultrafast volumetric imaging techniques. This will provide deeper insights into respiration, circulation dynamics, and rapid cell-cell communications such as calcium signaling, viscoelasticity, and energy dissipation at the alveolar level, all within a functioning lung.
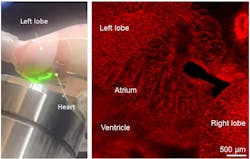
Moreover, our crystal ribcage can be used to image cardiac function and cardiac-pulmonary coupling in real time as well (see Fig. 5). Exploiting the scalability of our fabrication process and recent advances in ex vivo tissue maintenance, we plan to scale up the crystal ribcage for lung studies in large animals like pigs and transplant-rejected human lungs—enabling investigations in a more clinically relevant context.
ACKNOWLEDGMENT
The authors disclose that the data, figures, and content presented here are adapted from their own work previously published: R. Banerji et al., Nat. Methods, 20, 1790–1801 (2023); https://doi.org/10.1038/s41592-023-02004-9.
Rohin Banerji
Rohin Banerji is a Ph.D. student at the Center for Multiscale and Translational Mechanobiology (CMTM) based in the College of Engineering at Boston University.
Hadi Nia
Hadi Nia, Ph.D., is an assistant professor at the Center for Multiscale and Translational Mechanobiology (CMTM) based in the College of Engineering at Boston University.