Bioimaging: Is it possible to improve the performance of microscopes?
Bioimaging refers to the different techniques and systems used for in vivo, ex vivo, and in vitro imaging of biological samples, which allow researchers to obtain information at the molecular, cellular, or tissue level.
Microscopes are widely used by researchers for bioimaging to study subcellular structures, proteins, or organelles. For example, these images help us understand how cells and tissues are organized at the molecular level, identify and study substances in cells such as peptides or RNA, screen for potential drug candidates for the pharmaceutical industry, or serve as a real-time tool for intraoperative surgical margin assessment.
Microscope categories: brightfield and fluorescence
Brightfield microscopy is one of the oldest and most widely used techniques, thanks to its simplicity and easy handling. However, its use of white light can affect the resolution of images obtained (due to the effects of absorption, scattering, or deflection in the sample) and, because some biological specimens are thin and transparent, it can be difficult to achieve detailed, high- contrast brightfield images.
Fluorescence microscopy, which includes fluorescent widefield, confocal, two-photon, or light-sheet microscopy, uses fluorescent stains or proteins called “fluorophores,” which are typically introduced by a researcher. These molecules absorb a specific wavelength of light—the excitation wavelength—and emit a second, longer “emission wavelength.” This technique offers advantages, such as the possibility to detect different molecules simultaneously or to detect them even if not many are present—and single molecules can be imaged.
New illumination solutions
To increase the efficiency of the imaging process and achieve accurate results faster at higher resolutions, advances are being made in complete systems and components.
Lighting is one of the components that’s experienced progress during recent years. In different microscopy setups, light-emitting diodes (LEDs) surpassed halogen and arc lamps as one of the most popular components for illumination, because they offer many benefits: greater stability, repeatability, and reliability. They also come in discrete wavelengths and are more cost-effective, energy-efficient, and eco-friendly.
U.K.-based CoolLED developed novel high-intensity LED illumination systems for fluorescence microscopy and related tools, with solutions that include up to 16 LED channels in one box to cover a spectral range from 280 to 950 nm. They are working to reduce the damage in both fixed and live cells caused by phototoxicity and photobleaching—optimizing the illumination and enhancing the control of the LED systems and the through-the-lens (TTL) camera synchronization. These phenomena are caused by the absorption of photons from the illumination source and can have a detrimental effect during a microscopy experiment. CoolLED systems include several key features to optimize illumination of the sample and minimize photodamage.
Advantages of ultrafast lasers
Ultrafast lasers have opened the door for noninvasive methods relying on multiphoton excitation such as two-photon microscopy (TPM), second-harmonic imaging microscopy, or coherent anti-Stokes Raman scattering (CARS) microscopy.
These methods mainly depend on the nonlinear excitation resulting from the simultaneous absorption of two or more near-infrared (NIR) photons, which produces intrinsic optical sectioning like conventional confocal microscopy.
In the particular case of CARS, a third-order nonlinear process occurs when a sample is illuminated by two laser beams of different wavelengths that are synchronized in time and space: the “pump” and “Stokes” beams. As a result, the sample is excited by a wave-mixing process of both laser beams. The pump beam creates virtual stages in the sample, while the Stokes beam, which hits the sample simultaneously, stimulates the population of virtual levels. This simultaneous illumination creates a dense population of a vibrational sub-state of the ground state, which is very specific to the chemical properties of the sample under analysis.
Prospective Instruments developed their FSX-Dual laser for CARS microscopy. It is a <140-s, 100-MHz source with two emission wavelengths: one fixed at 1030 nm and the other tunable between 760 and 940 nm. This laser allows CARS microscopy, preferably at wavenumbers around 2850 cm-1, where lipids can be imaged label-free.
To synchronize the two output laser beams in time, one of the two beams is delayed via a time delay that both beams hit the sample at time zero and generate a CARS signal. This laser has been used to analyze xenografts in zebrafish, an increasingly used model system in cancer research because it provides a powerful and cost-effective platform for investigating various aspects of cancer biology—including tumor initiation, progression, metastasis, and drug responses.
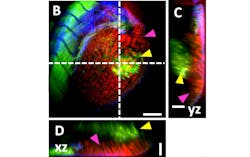
Figure 1 shows a three-dimensional (3D) volume scan of a xenograft: injected human osteosarcoma cells (OS143b). Before incubation, the injected cells were incubated overnight with the LuminiCell 670 fluorescent live-cell tracker (depicted in green), and then injected in the perivitelline space (PVS) of 3-day old zebrafish larvae. 24 hours later, the larvae were fixed and stained with Hoechst (depicted in blue) and Phalloidin-490LS (also depicted in green). The swim bladder provides a strong label-free CARS signal (depicted in red) around the xenograft.
While tunable lasers are widely used for different multiphoton microscopy applications, techniques that rely on a fixed-wavelength laser offer several advantages. This is the case for two-photon light-sheet microscopy, and Chromacity is developing single-wavelength laser sources with high average power and short-pulse duration to obtain cutting-edge images. This technique is designed to image intact organs, embryos, and organisms by detecting the fluorescence signal only at the in-focal plane—without the need for a pinhole or image processing. Sectioned images can be generated in real time and record a high-density 3D image stack within seconds, which represents a major advantage over traditional confocal laser-scanning microscopy.
Due to illumination through a sheet of light, optical power is spread across the whole sample—which reduces photodamage and stress of living samples. Chromacity’s model 1040 offers a ytterbium-doped fiber laser with emission at 1040 nm, pulse width <150 fs, and excellent beam quality for this type of application. Its high stability and high average power enable two-photon light-sheet microscopy to be carried out using any fluorescent agents with linear absorption in the 500-nm region—including RFP, YFP, GFP, Sytox green, fluorescein, and many others.
Figure 2 shows the image stack of a zebrafish embryo stained with eosin and fixed in agar gel inside a capillary, in a setup illuminated with a Chromacity 1040 laser. Zebrafish are mostly transparent and enable light-sheet imaging through the whole embryo. It’s then possible to create a stack of images at different depths to enable a 3D reconstruction of the specimen. In this image, the zebrafish head and tail are in focus and generating the strongest two-photon fluorescence signal.
Multimodal platforms
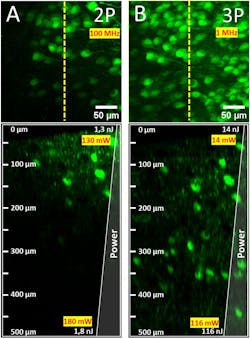
Multimodal nonlinear imaging platforms are an interesting way of using the distinct advantages of two-photon (2PM) and three-photon microscopy (3PM). In terms of penetration depth, 3PM facilitates deeper tissue imaging than 2PM (see Fig. 3), and it offers neuroscientists profound insights of structural and functional relationships. Other advantages of 3PM are its enhanced attenuation length in highly scattering tissue through longer-wavelength excitation, and a more precise excitation confinement by higher-order nonlinear excitation—which leads to a better signal-to-background ratio.
However, 3PM faces two challenges. First, because of water absorption, 3PM is limited to two specific excitation wavelengths (namely 1300 nm and 1700 nm) and only some synthetic dyes can be excited at these two wavelengths. Second, 3P excitation is more highly nonlinear and requires higher peak power than 2P. This makes it difficult to find the right setting between achieving a good signal and damaging the sample. Multimodal platforms combining a wavelength tunable 2P and 3P laser with a single microscope body enable 2P multicolor imaging with a broad fluorophore selection and deep tissue 3P imaging—covering the entire spectral window from 765 nm up to 1700 nm.
Currently available commercial 3P laser scanning microscopes require excessive utilities to operate and tend to be difficult to maintain, often requiring specialized equipment and expertise to be performed effectively. So Prospective Instruments created its MPC multiphoton multimodal imaging platform, a compact and flexible microscope system that enables interfacing with any femtosecond laser for 2P or 3P imaging. Its laser scan engine is designed to cover all wavelengths from 680 nm up to 1700 nm, so it is suited for different multiphoton imaging techniques or coherent Raman imaging, as well as 4-channel photomultiplier tube detection in either epi- or transmission mode to cover different sample thicknesses.
Automation of microscopy workflow
Another bioimaging trend is automated microscopy, which is becoming a commonly used process in applications that require mass testing. It’s being driven by artificial intelligence and deep learning. Opto GmbH, in Germany, specializes in automating microscopy and creates imaging modules that can be integrated into more complex systems for continuous monitoring of samples—without the need to remove specimens from the device. These solutions can minimize the risk of human error and provide reproducible results. Combining Opto’s imaging modules with an open software platform allows users to create their desired solution individually and tailored to their needs.
Novel spectroscopy methods
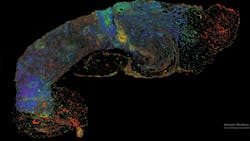
A new laser-based microscope technique, photon absorption remote sensing (PARS), permits cellular-level resolution of unstained fresh, frozen, and fixed tissues. This optical absorption microscopy method unlocks and identifies the unique absorption of biomolecules by collecting and analyzing multiple optical channels. This redefines the boundaries of optical imaging by producing unprecedented cellular and molecular data. The company illumiSonics developed the first all-optical imaging system based on this technique, and it is capable of instant molecular imaging—without contact or damage to tissue (see Fig. 4). It has the potential to disrupt the pathology workflow because it replaces the 100-year-old staining process and enables multiplexing (multiple virtual stains) and molecular diagnostics from a single sample for rapid diagnosis and informed treatment. PARS microscopy can facilitate digital pathology with enhanced biomarker analysis and AI-based diagnostics to improve the speed and accuracy of cancer diagnosis.
What’s ahead?
Several great developments in systems, components, and methods are leading microscopy and bioimaging to revolutionize biomedical research and clinical diagnostics with unprecedented precision and detail. Integrating artificial intelligence and machine learning will enhance image analysis, and enable more accurate interpretations and predictions. These innovations promise to accelerate discoveries in disease mechanisms, drug development, and personalized medicine—and, ultimately, transform our understanding and treatment of complex biological systems.
About the Author
Antonio Castelo
Antonio Castelo is Technology Manager for Bio-Medical and Lasers at the European Photonics Industry Consortium (EPIC).