Ultrafast lasers generate pulses shorter than about 1 ps, transferring energy to electrons before they interact with their environment. With high intensity, for example, an electron can receive energy from two photons before losing any of that energy in molecular vibrations or interactions with other electrons.1 That unique interaction introduces distinctive capabilities in microfabrication and direct manipulation of living cells.
Femtosecond lasers minimize heat transfer to ablate material without melting or re-solidification, leading to very clean microfabrication. In addition, when focused to a spot a few microns across, the beam can excite electrons with two low-energy photons in place of a single photon with twice the energy. This happens only at high intensity, limiting interaction with the target material to the focal region.
The first femtosecond laser was invented in the 1970s, but biologically relevant advances continue, including two recent representative developments—one in microfabrication and one with direct stimulation of living cells.
Building a trap
When a photopolymer is exposed to light of a specific wavelength, monomers join, creating a polymer. Two-photon polymerization (TPP) occurs when the material is exposed to femtosecond laser pulses of twice the polymerization wavelength. As this only happens near the focal point, TPP produces very fine features.
Professors Haibo Yu and Lianqing Liu of Northeastern University (Shenyang, China) and their colleagues used TPP to create self-assembled structures to mechanically trap individual cells.2 They created “micropillars” by exposing one location in a photopolymer to 240 fs pulses from a 1030 nm laser operating at 200 kHz. By varying laser power and moving the focus, they produced micropillars with diameters from about 2 to 7 µm and heights from 5 to 40 µm. They also created patterns of micropillars, such as isosceles triangles with sides of 5 µm and 10 µm or a “butterfly” pattern of four micropillars at spacings of 10 to 20 µm (see Fig. 1). After light exposure, unpolymerized photoresist is washed away with a developer and then dried. As the developer evaporates, capillary forces pull the micropillar tops together, creating three-dimensional (3D) cages.
The intention was to create structures to hold individual cells without constant active intervention. Optical traps hold individual cells, but there is no easy way to retain multiple cells or hold them for a long time. After some proof-of-principle tests, Yu and Liu used an optical trap to slide individual mouse fibroblast cells inside the 3D butterfly cage. The butterfly cage retained cells even during fluid flow, such as those that would take place during nutrient addition or medium replacement, and with no continuous external force. This new capability now enables longitudinal studies of individual cells.
So, ultrafast lasers micromachine structures useful for biological studies, but they can also directly modify the behavior of living cells.
Cellular control in a flash
Ultrafast lasers provide the peak intensity required for multiphoton microscopy, in the same way as they do with TPP. Here, femtosecond lasers excite fluorescent labels at twice their nominal absorption wavelength. Previously, some researchers had noticed that multiphoton microscopy sometimes stimulated transient Ca2+ signals.3 Professor Hao He of Shanghai Jiao Tong University in China and his colleagues at several other international institutions pursued those investigations and discovered an ultrafast laser method to specifically and deterministically stimulate Ca2+ influx in a variety of cell types.4
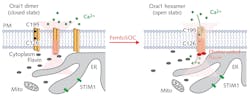
Calcium regulates a wide range of cellular processes in all eukaryotic cells. There are a number of different mechanisms that regulate and effect the transfer of Ca2+ across the cell membrane. One of those mechanisms is the store-operated calcium (SOC) entry, in which one protein—STIM1—reacts to the Ca2+ concentration within the endoplasmic reticulum and binds with another protein—Orai1—to create a channel allowing calcium influx to the cell. Although the details of the Orai1 channel formation aren’t yet well understood, the consensus understanding is that the calcium channel opens when the Orai1 proteins assemble in a tight hexamer configuration.
Because calcium signaling is critical to many biological processes, researchers have developed genetic modifications that allow calcium influx to be optically controlled. Optical stimulation is the least invasive technique to activate specific cells, but genetic modification is about as invasive as it gets. He’s team found they could activate Ca2+ channels in wild-type cells by illuminating the cellular membrane with 700 nm light delivered in 100 fs pulses from a 2 mW femtosecond laser.
Isolating the mechanism
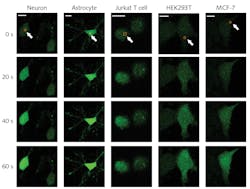
To investigate this effect, He’s team first incubated cells with a fluorescent calcium marker and imaged the culture with a confocal microscope using a 488 nm laser source. Once a target cell was identified, the femtosecond laser was scanned over a small region of the cell membrane—typically a 2 × 2 µm area. Confocal microscope images quantified changes in the cell’s internal calcium concentration. The researchers saw an influx of calcium originating at the exposure site and spreading throughout the cell, as can be seen for various cell types in Figure 2.A comprehensive set of experiments isolated the mechanism. By performing the procedure in environments both with and without external Ca2+, they verified that the calcium diffusion came from the external environment and not an internal Ca store. Using various techniques to prevent the action of the Orai1 and STIM1 proteins, they verified that the calcium was entering through the Orai1 channel with no involvement of the STIM1 protein. With FRET labeling, they confirmed that femtosecond laser exposure stimulated the formation of a tight Orai1 hexamer structure.
They then scanned the femtosecond laser over a wavelength range of 700 to 960 nm and noted the highest Ca2+ influx at the shortest wavelengths—far from water’s peak absorption, indicating photothermal effects were not a likely cause. The two-photon absorption spectrum also matched that of flavin, hinting that flavin excitation might be responsible for Ca channel formation. By introducing agents that inhibited that excitation, they confirmed that photoexcited flavins trigger the opening of the Orai1 channel, as illustrated in Figure 3.Those experiments, along with several others, confirmed that exposure to appropriate femtosecond laser pulses triggers calcium influx through the store-operated calcium channel (SOC), leading to the method’s abbreviated name: femtoSOC. To demonstrate femtoSOC’s utility, He’s team induced calcium influx specifically through SOC in a range of different cell cultures, including neurons, astrocytes, and MCF-7 breast cancer cells. They also demonstrated the ability to trigger calcium influx in single neurons in living mice, opening a novel path to investigating neural interactions.
Professor Hajime Hirase of the Center for Translational Neuromedicine at the University of Copenhagen in Denmark has been developing optogenetic resources for stimulating astrocytes to enhance their Ca2+ levels. He recently demonstrated that astrocyte Ca2+ elevation leads to enhanced memory performance in mice.5 Hirase, who was not involved in the femtoSOC development, appreciates its potential for noninvasive investigation of cognitive processes, in that femtoSOC “obviates the need for transgenic mice or AAV inoculation to manipulate astrocytic calcium.” He believes this stimulation modality will be even more valuable as more precise manipulation of activation and deactivation of neuronal activity is achieved “because brain function relies on the action potential timing and rate of neurons.”
He’s team is proud of the ability to directly control SOC channels without optogenetics.
“This is the sole technology to control an ion channel by light with high specificity without optogenetics, although elucidating the mechanism is quite challenging,” he said, but the possibilities are tantalizing. “We are working on a series of applications of this work, including the treatment of Alzheimer's disease.”
REFERENCES
1. R. R. Gattas and E. Mazur, Nat. Photon. (Apr. 2008); doi:10.1038/nphoton.2008.48.
2. P. Li et al., Opt. Express, 29, 7 (Mar. 2021); doi:10.1364/oe.420033.
3. O. Garaschuk et al., Nat. Protoc., 1, 1 (2006); doi:10.1038/nprot.2006.58.
4. P. Cheng et al., Cell Res., 0, 1–15 (2021); doi:10.1038/s41422-020-00463-9.
5. Y. Iwai et al., Front. Neural Circuits, 15 (2021); doi:10.3389/fncir.2021.658343.

Richard Gaughan | Contributing Writer, BioOptics World
Richard Gaughan is the Owner of Mountain Optical Systems and a contributing writer for BioOptics World.