Optogenetics: Lighting up the brain enables surprising discoveries
Optogenetics combines optics, genetics, and virology to allow researchers to use light to control brain cells genetically engineered to respond to specific wavelengths. It’s revolutionized neuroscience by enabling researchers to shine light into the brains of animals to explore neural circuits and make surprising discoveries.
The basic concept at the heart of optogenetics is that many cells found within nature respond to light and, when activated with light, charged ions flow into the cells to create an electrical potential difference.
One light-sensitive protein from algae, an opsin akin to a tiny solar cell, was discovered to be biocompatible with brain neurons in 2005. This very first optogenetics opsin, channelrhodopsin-2 (ChR2), passes electrical charge in response to blue light. And since opsin DNA can be inserted into a virus and then injected into neurons, brain cells can be “painted” to code the protein and become light-sensitive.
“In the early days of optogenetics, it was often used to verify things we already knew,” says Ed Boyden, one of the pioneers of optogenetics and a professor of neurotechnology at MIT. “But those days are over and researchers are making more and more totally surprising discoveries.”
Optogenetics tool advances
Optogenetic tools have come a long way during the past 10 years, especially if you consider that since the dawn of optogenetics we’ve seen the emergence of precision genome engineering with CRISPR technology.
“All of this is now available—to improve genetically targeted control—whether it’s genome editing and RNA technology, better virus delivery techniques, or complete cell type atlases,” says Brian Chow, an associate professor of bioengineering at the University of Pennsylvania. His lab invents optical tools to manipulate and monitor cellular physiology to explore how cells make decisions in response to external cues.
“There are so many advances from diverse fields that can be leveraged,” he says. “I think we’ll see their trickle-down effect moving forward. For example, my lab has benefitted from innovations in cryogenic electron microscopy and computational protein structural prediction to build better optogenetic protein tools. I won’t say we have everything we need, but optogenetics tools today are sufficiently diverse, and powerful enough now for most people to study the problems they’re dreaming of.”
All optogenetic tools are natural photosensory proteins, so Chow spends a lot of time talking to microbiologists who are sequencing random fungi or critters found in the ocean’s depths, and others studying photoreceptors from these organisms to try to understand how these photoreceptors regulate circadian rhythms or pathogenesis.
Chow’s lab is currently working on cell sensing and intracellular biology and signaling. “This work has lagged a few years behind the opsin and excitability work in neuroscience that launched the field, but many of the questions we want to ask are mediated by intracellular signaling,” he says. “Between excitability, signaling, and control over transcriptional and epigenetic regulation, the world’s your oyster for probing a cell’s dynamics.”
But frontiers in optogenetics tools remain, Chow points out, in particular in vivo and the need to redshift tools to be better in line with the optical window for deeper tissue penetration.
“Many people working in photodynamic therapy and molecular imaging remind me that redshifting for a biological chromophore is still short wavelength compared to other optical techniques further in infrared (IR), and they work deeper into tissue than we normally think of as being possible with optogenetics,” he says. “So there’s still a frontier of how do we get deeper into tissue? Either optically, other forms of electromagnetic radiation, or through in vivo surgical access? From a technology perspective, this is where there’s still a great need.”
Chow’s lab has tried to go deeper into IR, but one of the challenges in optogenetics is that chromophores and the proteins are naturally derived. “Living systems have a fairly limited bandwidth of light they use—in the near-IR to the ultraviolet (UV),” he explains. “And near-IR is maybe just far red around 720 nm and below. If you’re talking to medical imaging people about near-IR, they’re thinking of longer-wavelength laser lines, closer to 1000 nm.”
The challenge here is there aren’t many photoreceptors useable to get that far out, and those existing within nature aren’t endogenous to the mammalian body. “This ends up meaning you’re expressing a protein within a cell that theoretically is very efficient at absorbing near-IR, but often most of the protein is blind or optically inactive,” Chow says.
To get a better understanding of these issues, Chow’s lab is exploring the underlying fundamental process of how these cofactors are taken up and bind to the proteins so they can improve the efficiency so most of the protein can see, as opposed to being blind.
They’re also interested in whether or not they need to limit themselves to natural cofactors, and have experimented with binding nonmammalian cofactors. “You can imagine if you have bonafide cofactors in the IR because they’re made of the transition metal complexes, you could get out quite far and with sharper absorption bands than bioorganic cofactors, which would be valuable because spectral multiplexing is still quite hard,” he adds.
And there are still novel modalities. Chow’s lab recently discovered a blue-light responsive protein that showed a surprising temperature responsivity—meaning they can combine light and heat.
Now they’re wondering if they can rely on only the temperature responsivity of this probe without the blue light. “It’s probably one of the most promising routes we’re currently working on to get really deep into tissue with our collaborators in Lukasz Bugaj’s lab at the University of Pennsylvania,” Chow says.
But there are other promising ideas within the field, such as sonoluminescence, which gives off blue light where most photoreceptors absorb, so you can imagine getting really deep because it’s due to ultrasound. “Another concept is upconverting nanoparticles to get further into the IR,” Chow adds. “All of these approaches show promise, but there aren’t enough hours in the day for our lab to tackle them all.”
Chow emphasizes how important the commoditization of high-power light-emitting diodes (LEDs), their cost-effectiveness, and growing spectral range were during the past decade. “It’s much easier to expand the excitation capabilities of a microscope or spectrophotometer, or to build custom optoelectronic hardware to meet the needs of your experiment—especially when you also have access to low-cost 3D printers to make optomechanics,” he says. “It’s so simple, yet profoundly enabling. For example, we and collaborators have built low-cost multimode plate readers, 96-well plate illuminators with integrated detectors, all led by undergraduates.”
Single-cell and holographic optogenetics
A growing number of research teams are using a two-photon laser approach, pioneered by Valentina Emiliani’s group at the Institut de la Vision in France, on single cells to give every cell its own code and project holograms into the brain.
“Researchers often use optogenetic studies to drive every cell in the same way, but in reality that’s not how the living brain works,” Boyden points out. “Holographic optogenetics is very exciting because it gets closer to how the brain works and, although it’s still early days, you can see what’s coming down the pipeline.”
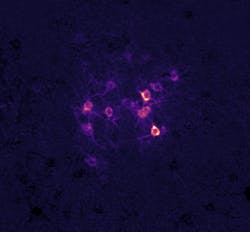
Patrick Kanold’s lab at Johns Hopkins University is exploring how sound experience shapes later sound perception (see figure). And being able to alter the activity of cells with optogenetics paired with the ability to precisely shape light with holography allows them to manipulate the activity of identified single cells within behaving animals.
“It lets us ‘crack the neural code’ by stimulating groups of neurons in behaving mice to see if we can alter the animal’s percept,” says Kanold, a professor of biomedical engineering. “We can thereby link the activity of groups of neurons to sensory percepts, and then investigate how changing experience alters this ‘code,’ e.g. how is the set of neurons that we need to stimulate to evoke a percept different after a particular sound experience.”
Inverse optogenetics?
One of the Holy Grails of neuroscience is to build the opposite of optogenetics: to measure the voltage of the neurons using light. This is an area Boston University (BU) researchers are working on, and they’ve shown you can image the brain’s voltage with red light and control the brain’s neurons with blue light.
Xue Han, professor of biomedical engineering at BU, recently led a study where they used a mutated optogenetic protein, SomArchon, as a fluorescent voltage indictor. When red light was shined on an awake mouse brain, neurons expressing SomArchon emitted IR light in proportion to their voltage. By collecting the light on a camera, the voltage of many neurons can be imaged at the same time. In that way, the scientists can see how different neurons fire with respect to brain rhythms or in relation to behavior. By expressing a blue-light driven optogenetic molecule, CoChR, in the same neurons, it was possible to drive them with blue light and image them with red light.
“I’m intrigued by the possibility of an all-optical input/output strategy,” says Boyden. “It may enable imaging of the brain’s tissue without needing to put any probes into the brain. This would be a more stable and less damaging approach.”
Taking on Alzheimer’s via light and sound
One of the most surprising discoveries via optogenetics so far was by Li-Huei Tsai, director of MIT’s Picower Institute for Learning and Memory, as well as a professor of neuroscience at MIT. In 2016, her group discovered that driving gamma waves (40 Hz) within the brains of mice that model human Alzheimer’s disease can cause the brains to recover. They went on to simulate the effect without optogenetics by simply combining flickering LED light at 40 Hz with sound stimuli in the form of 40 Hz tone pulses.
Tsai and her group stimulated gamma oscillations at 40 Hz using optogenetics to control the activity of genetically modified neurons by shining light on them. These neurons, known as interneurons, synchronize by spreading gamma activity to excitatory neurons.
After an hour of stimulation at 40 Hz, levels of beta amyloid proteins within the hippocampus dropped 40 to 50%. Interestingly, stimulation at other frequencies didn’t show the same result.
Turns out, light stimulation directed at the hippocampus substantially reduces amyloid plaques and cleans up Alzheimer’s disease within the visual cortex of mice brains.
So the researchers wondered: could this be done as a completely noninvasive technique to help people as well? To do this, they created a brain wave movie that combines light and sound at the magical 40 Hz. “The mice watched the movie for an hour each day for a week, and afterward the amyloid had decreased,” says Boyden.
This technique is so promising, Tsai and Boyden launched a startup, Cognito Therapeutics in 2016, and are currently in multiple large-scale clinical trials with people diagnosed with mild to moderate Alzheimer’s disease.
“It’s exciting to go after one of the most intractable diseases of our time,” says Boyden. “All of the Alzheimer’s treatments available today just help with symptoms, rather than slow or stop the disease. Our noninvasive approach, if it passes clinical trials, could be done right in your home.”
Gene therapy for blindness
Treating blindness is another realm where optogenetics is providing surprising results, and several groups are currently working on it. Bionic Sight, founded by Sheila Nirenberg, a professor of physiology and biophysics at the Weill Medical College of Cornell University, is exploring an investigational gene therapy for retinitis pigmentosa, a genetic disease.
Nirenberg’s approach involves injecting an optogenetic protein, which is activated via a neural coding device. “Her group helped unravel the neural code of the retina, which is how our retinas translate the world into signals our brain can understand,” says Boyden. “They’re using a molecule our group discovered in 2014 and, if their trials go well, it will open the door to more.”
Impressively, the first four patients with complete or near-complete blindness participating in a clinical trial can now see light and motion, and two can detect the direction of motion.
“I applaud the brave souls analyzing whether optogenetics can directly help human beings—it takes real guts to do it,” says Boyden.
About the Author
Sally Cole Johnson
Editor in Chief
Sally Cole Johnson, Laser Focus World’s editor in chief, is a science and technology journalist who specializes in physics and semiconductors.