I will always remember the first time I peeled off a bit of onion epithelium and placed it under a microscope. Very cool. I could actually see the cells. As a high school student, it wowed me. Then I added a drop of methylene blue. Astounding! Over a background of lighter blue, the dark-blue cell boundaries jumped out, and the nuclei appeared as small blue spheres. Such onion-peel preparations lured more than one budding biologist into the field. But what if we could see even more, delve even deeper into tissues, and do it all without using any dye or label?
“Labels are great,” says Lev Perelman, associate professor at Harvard University and director of the Biomedical Imaging and Spectroscopy Laboratory at Beth Israel Deaconess Medical Center (Boston, MA). “But there is the suspicion that you affect cell function. You don’t know if your observations are from proper cell functions or from the label.” Moreover, it’s pretty easy to get dyes and labels into dead cells, but some live cells put up a fight. In short, biologists and medical scientists want to look at living cells without putting anything in them or on them.
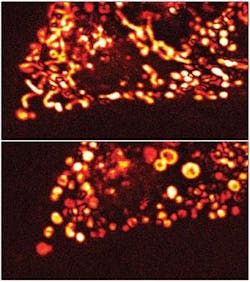
Like many scientists, Perelman wanted to see what lies behind cancer. He and his colleagues developed light-scattering microscopy in hopes of improving early detection of cancer and doing it in a person. With this technology, he could see epithelial cells—common starting points of cancer—but not the organelles inside of them. He wanted to see more.
Seeing organelles inside cells usually requires a label because these structures provide little contrast on their own. To avoid using any dye, Perelman and his team developed confocal light absorption and scattering spectroscopic (CLASS) microscopy, which is described in the Oct. 30, 2007, Proceedings of the National Academy of Sciences.1 CLASS uses light-scattering spectroscopy to detect organelles and confocal microscopy to gather spatial information. To illuminate a sample, Perelman tried a couple of approaches, including using a supercontinuum laser (such as Fianium’s SC-450-2), which provides illumination at wavelengths from 450 to 2000 nm. A thermoelectrically cooled detector gathers the light reflected from the sample and sends it to a spectrograph (Acton Research SpectraPro 150). The transmitted light goes to a confocal microscope.
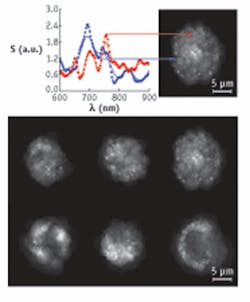
Instead of adding a contrast agent, CLASS depends on physical differences between organelles—specifically, their average refractive index when illuminated with light of various wavelengths. “How does the refractive index change along the wavelength axis?” Perelman asks. That change is the key to distinguishing organelles. “More change gives you more contrast, a more specific ‘marker,’ ” he explains.
Using human bronchial epithelial cells, Perelman and his colleagues could distinguish mitochondria, which are 700 to 1100 nm long, and peroxisomes, which have diameters of about 400 nm. The sensitivity, though, seems to be even better, maybe as low as 10 nm—below the diffraction limit of light. This is possible because CLASS creates a refractive-index curve across a continuum of wavelengths and then compares curves between organelles to distinguish one from another.
An indirect route
Sometimes advances in label-free imaging come to biology by good fortune. That’s how Laurent Cognet, research associate at the Université Bordeaux, France, brought light-induced scattering-around-a-nanoabsorber (LISNA) microscopy to label-free, live-cell imaging.“The idea was to develop a method to detect absorbing nano-objects instead of fluorescent probes,” Cognet says. As described in the Oct. 17, 2007, Optics Express, LISNA uses an exciting beam—a Nd:YAG at 532 nm or an argon laser at 514 nm—that is modulated, usually at 700 Hz.2 This laser’s energy gets absorbed by the target. That absorption heats up the target, which changes its nearby refractive index. A probe laser—632.8 nm HeNe—detects the object through the field scattered by the induced refractive-index profile. The exciting beam’s modulation creates a frequency component in the scattered light. As Cognet explains, “The probe laser sees the change in refractive index, and the detected component at the modulated frequency is very sensitive.”
When Cognet turned this technique away from particles—gold nanospheres as small as 1.4 nm—and aimed it at cells alone, he too could image mitochondria with detail in live cells in different morphological states.
Biologists will always want to see more and scientists will keep looking for ways to unveil nature in its untouched form. Through the combination of so many scientists seeking these new ways to see, we’ve come a long way from simply staining onion cells with methylene blue.
REFERENCES
1. I. Itzkan et al., PNAS 104(44) 17255 (2007).
2. D. Lasne et al., Optics Express 15(21) 14184 (2007).
About the Author
Mike May
Contributing Editor, BioOptics World
Mike May writes about instrumentation design and application for BioOptics World. He earned his Ph.D. in neurobiology and behavior from Cornell University and is a member of Sigma Xi: The Scientific Research Society. He has written two books and scores of articles in the field of biomedicine.