MICROSCOPY/FLUORESCENCE IMAGING/FIBER LASERS: Fiber for two-photon microscopy
Titanium sapphire lasers have become the de facto standard for multiphoton microscopy, even though many experiments require neither tunability nor high output. For these situations, less costly, less complex fiber lasers are proving highly capable for cell imaging and other applications.
ByMarion Lang and Tilman Franke
Since its debut in 1990, multiphoton (or two-photon) microscopy has become a very popular technique for cell imaging.1 The nonlinearity of two-photon excitation results in intrinsic 3-D resolution, also called optical sectioning. An advantage of this approach is the greater penetration depth of the infrared light used for excitation (compared to shorter wavelengths), which therefore enables imaging of thicker samples.
This infrared laser light—pulsed, for the excitation of fluorescent labels—is most frequently supplied by titanium sapphire (Ti:Sa) lasers. While Ti:Sa lasers are appreciated for their wide tuning range (710–990 nm) and their high output powers (300–3000 mW, depending on wavelength), they are complex systems that require active cooling. An upcoming alternative are erbium (Er)-doped fiber lasers operating at 780 nm. Fiber lasers are less costly and complex, more compact, and they consume much less power than Ti:Sa lasers. They are also stable, push-button systems that are insensitive to changing environmental conditions. These features make them suitable for routine use in research labs. Today, fiber lasers are ready for many multiphoton applications and can replace bulky Ti:Sa lasers in experiments that require neither tunability nor high output powers—a situation that is strikingly common.
Assessing the fiber alternative
A team of researchers—from Till Photonics, the BioImaging Center of the Ludwig Maximilian University (Munich, Germany), and Toptica Photonics—has analyzed the potential of a fiber laser for two-photon microscopy and compared the results to those they obtained with a conventional Ti:Sa laser. The fiber laser (FemtoFiber pro NIR, Toptica Photonics) is a compact, frequency-doubled Er fiber laser operating at 780 nm with an output power of 140 mW.
To conduct the assessment, the team placed the portable fiber laser on an optical table along with a commercial Ti:Sa laser and an inverted microscope (iMIC, Till Photonics). They coupled both lasers to the microscope and used a flip-mirror to switch between them. A telescope in each path ensured the same beam diameter on the scan mirror. Attenuation was supplied on the Ti:Sa laser by an electro-optic modulator (EOM), and for the fiber laser by a graduated neutral density filter. Both lasers operate at a repetition rate of 80 MHz and have a nominal pulse length of 100 fs (see Fig. 1).
Avoiding photodamage
Applied to such specimens as single cell layers, tissue slices, and embryos up to whole animals, two-photon excitation is prized for its low photodamage, making it useful for live-cell imaging, and high penetration depth, making it appropriate for imaging of thick samples.
In fact, reduction of photodamage overall—compared to single-photon excitation—is one of the major advantages of multiphoton microscopy, which confines photodamage to the focal region. Even so, though, at higher power levels multiphoton microscopy—especially when used for live-cell imaging—is limited by both photodamage and phototoxicity. Some researchers have reported that excitation intensities above 10 mW at the specimen plane lead to severe damage after only a few scans.2 Others state that only power levels less than 2.5 mW at the specimen plane can be considered “safe” for typical experiments.3 Thus, the power levels of Ti:Sa lasers are not necessarily an advantage, as no biological tissue withstands these powers directly. And even at the lower output powers of currently available fiber lasers, one has to choose the right parameters for imaging in order prevent photodamage.
The versatility of 780 nm
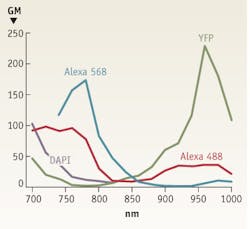
Although one might logically conclude that the peak of two-photon absorption is equal to twice the one-photon absorption wavelength, instead, it is actually blue-shifted for many fluorophores. So for example, with maximum single-photon excitation at around 570 nm, Alexa 568 has a two-photon absorption maximum of 780 nm—not 1140 nm. Furthermore, multiphoton absorption spectra are typically much broader than one would expect from the corresponding one-photon spectra.
A consequence is that dyes with different single-photon excitation wavelengths can be conveniently excited by two photons at a single wavelength.5 As a matter of fact, many dyes—including the popular Alexa 488, Alexa 568, and Alexa 594—can be efficiently excited at around 780 nm (see Fig. 2). Thus, the output wavelength of frequency-doubled Er-fiber lasers is suitable for exciting most dyes. DAPI (with a two-photon excitation maximum around 700 nm) and the fluorescent protein YFP (maximum at 960 nm) are among the few dyes that cannot be optimally excited with lasers operating at 780 nm.
Experiment results
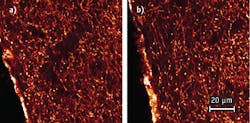
The research team directly compared image pairs that were recorded with the two lasers. First, they tested mouse olfactory bulb tissue with axons from receptor neurons stained with Alexa 488, which serves as an example of dyes that are optimally excited at 780 nm. For these measurements, the Ti:Sa was tuned to 780 nm so that both lasers operated at the same wavelength suitable to excite Alexa 488.
The image quality obtained with both lasers turned out to be identical (see Fig. 3). Note that the brightness values of all displayed images are scaled linearly according to their individual minimum/maximum values. To achieve identical intensity in the images required setting the fiber laser to 10 mW at the focal plane and the Ti:Sa to approx. 16 mW. The ability of the the Ti:Sa laser to reach only ~60% of the excitation efficiency of the fiber laser is due to a longer pulse length of the Ti:Sa at the sample. This finding is supported by a separate experiment, in which the team determined the pulse length in the focus of the objective to be 424 fs for the Ti:Sa and 210 fs for the fiber laser.
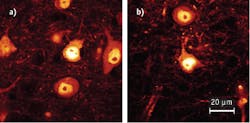
In a second experiment, the team imaged slices of mouse brain tissue in which ~1% of all nerve cells express YFP. YFP represents the “worst-case scenario” for the fiber laser operating at 780 nm, when compared to a Ti:Sa that is tuned to the ideal wavelength for YFP excitation (experimentally determined to be at 950 nm). For this experiment, both lasers were set to give 10 mW in the focal plane of the objective.
The resulting images show similar image quality, especially in the bright cell bodies (see Fig. 4). As expected, the fiber laser yields a lower contrast because the excitation of YFP at 780 nm is less efficient than at 950 nm. Still, the fiber laser performs reasonably well even in this setup. The brighter background at 780 nm is due to auto-fluorescence; the team found that this background signal was also present when the Ti:Sa was tuned to 780 nm.
Overall, the experiment results indicate that a fiber laser is indeed capable of replacing Ti:Sa lasers as a light source in many multiphoton applications. And being compact, stable, push-button systems, fiber lasers offer many advantages with respect to handling and operation.
REFERENCES
1. W. Denk et al., Science 248, 4951, 73–6 (1990).
2. W. Koester et al., J. Biophys. 77, 2226–2236 (1999).
3. A. Hopt and E. Neher, J. Biophys. 80, 2029–2036 (2001).
4. http://www.drbio.cornell.edu/cross_sections.html.
5. C. Xu et al., Bioimaging 4, 198–207 (1996).
Marion Lang is technical marketing manager, Toptica Photonics AG (Munich, Germany; www.toptica.com), and Tilman Franke is multiphoton product manager, Till Photonics GmbH (Munich, Germany; www.till-photonics.com). Contact Dr. Lang at [email protected].
More BioOptics World Current Issue Articles
More BioOptics World Archives Issue Articles