INSIDE INSTRUMENTATION: The many approaches and applications of biosensing
A middle-aged man leans forward, rests the bridge of his nose against a desktop-size device and waits a few seconds. When he hears a small beep, he pulls back and reads his blood glucose level on a screen—all without a single prick. That’s a futuristic vision of a device that hopes to improve the lives of people with diabetes by reading glucose levels from the eye. And it’s just one of many ongoing advances in biosensors.
In Gerard Coté’s biomedical engineering laboratory at Texas A&M University (College Station, TX), he and his colleagues already envision such an eye-based monitor. The concept depends on polarized light from a low-powered laser, which passes through the aqueous humor that fills the front of the eye. The magnitude of rotation in the polarized light as it passes through the fluid correlates with the glucose level, which is the nearly the same in the aqueous humor and the blood.
Eventually, Coté expects to use two wavelengths of light in this device. The eye will probably move some during the reading. Using the difference between the two wavelengths can eliminate that ‘noise.’
“We’re trying to build an instrument that is equivalent to a finger prick in measuring glucose,” Coté says. So far, tests suggest that his technology could be about as sensitive as measuring glucose levels in a blood sample, but Coté needs to confirm this on rabbit eyes before moving to humans.
Beyond being noninvasive, Coté’s device would also allow diabetic patients to monitor their blood-glucose levels more often. A patient wouldn’t have to worry about running out of fingers to prick. In addition, Coté believes that his system could be more economical in the long run.
More from multiplexing
When researchers explore biological samples, they often use labels, such as fluorescent markers. Those markers, however, might change how a reaction works, so researchers would rather avoid them. The pursuit of label-free bio research makes up some of the motivation behind a sensor being developed in Marko Loncar’s laboratory at Harvard University (Cambridge, MA).
This optical biosensor array relies on a photonic crystal nanobeam cavity, which is fabricated in a silicon wafer and capped with protective polymers. Qimin Quan, a graduate student in Loncar’s lab who is working on this biosensor, describes this component as a “nanoscale mirror on a chip that traps light.” For now, Quan uses 1550 nm light, which is commonly used in the telecommunications industry, but he adds that “you can use any wavelength in principle.” (see Fig. 1.)
On a chip that is, say, a centimeter or two across, this biosensor could contain thousands of nano-cavities. Sample fluids flow through the chip, which measures the change in refractive index. So this biosensor could detect molecules like drugs, or even a virus. In addition, it can look for different molecules with each nano-cavity. Consequently, one chip could detect thousands of different things.
Specific detection, however, requires modifying each nano-cavity. For example, an antibody could be added to attract particular molecules. As Quan points out, such modifications can be done with microarray technology, similar to spotting probes on a DNA chip.
Some companies also offer instruments that use light to simultaneously explore a range of biological samples. For example, the ProteOn XPR36 from Bio-Rad Laboratories uses surface plasmon resonance to create what Laura Moriarty, this instrument’s product manager, describes as “an optical biosensor that can capture 36 pieces of information at once.” (see Fig. 2.) For instance, a researcher can explore the kinetics of six proteins interacting with six receptors. Moriarty also points out that this instrument can be used to find the most sensitive bonding areas on an antibody. She adds that Bio-Rad makes a collection of application-specific chips to enable researchers to easily capture tagged proteins from a sample—including one to capture biotinylated proteins and another to capture HIS tag proteins—that work in this system.Synchronizing systems
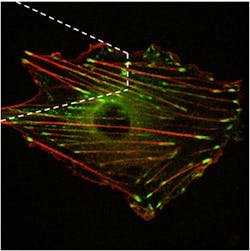
In some cases, researchers face a struggle to even capture the measurement-related light. For instance, Andreea Trache—assistant professor at the Texas A&M Health Science Center in College Station—explores how cells react to mechanical stimulation, such as the required expansion and contraction of blood vessels. Trache provides the mechanical stimulus to a cell with the tip of an atomic force microscope (AFM) and watches the changes in cells using total internal reflection (TIRF) or fast-spinning disk confocal microscopy (see Fig. 3).Specifically, this process stimulates the cortical actin, which is one of the main cytoskeletal proteins; basically, the structural frame inside a cell. To stimulate the actin, Trache uses a fibronectin-functionalized AFM tip. Fibronectin, an extracellular matrix protein, binds to receptors called integrins on the outside of the cells. Inside the cell, integrins connect to actin via focal adhesion proteins, such that the AFM mechanical stimulus is transmitted to the cytoskeleton.
Trache can watch for specific changes in actin filaments by labeling them with green fluorescent protein (GFP). To keep from adversely affecting the cells, though, Trache and her group express the GFP at low levels. She records the action with QuantEM 512 SC EMCCD cameras from Photometrics (Tucson, AZ). “This camera gives us high contrast images with a short exposure time,” Trache says. Since the experiments usually last 80 minutes, she limits the fluorescence excitation laser light and quickly records the three-dimensional actin images. She adds that this camera was easy to be synchronized with the spinning disk scanner of the confocal microscope. “We tested several cameras,” she adds, “and these seem to do the best job for all the features that we’re looking for.”
These features reveal fundamental biology for now, but could lead to clinical applications. “We are trying to understand the basic biology of the mechanical transduction process,” Trache explains. “We hope that our research will explain the mechanism of force transmission in the blood vessel wall. For example, in hypertension, changes in the vessel wall’s ability to constrict and dilate substantially impact vascular control and reactivity.”
Like these vessels, the definition of biosensors changes continually; but the field of biosensors keeps expanding—and never contracts.
About the Author
Mike May
Contributing Editor, BioOptics World
Mike May writes about instrumentation design and application for BioOptics World. He earned his Ph.D. in neurobiology and behavior from Cornell University and is a member of Sigma Xi: The Scientific Research Society. He has written two books and scores of articles in the field of biomedicine.