FLUORESCENCE MICROSCOPY: Nobel Prize honors super-resolution microscopy pioneers
On October 8, 2014, the winners of the 2014 Nobel Prize in Chemistry were honored for their work to overcome the resolution limit of standard optical microscopy, which Ernst Abbe famously stated in 1873 was 0.2 μm, or 200 nm (the shortest wavelength of visible light).
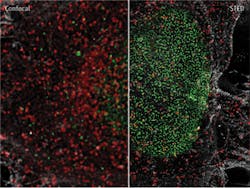
The honorees—Eric Betzig, Ph.D., a group leader at Howard Hughes Medical Institute's Janelia Research Campus (Ashburn, VA); Stefan W. Hell, Prof. Dr. Dr. h. c. mult., a director with the Max Planck Institute for Biophysical Chemistry (Göttingen, Germany); and William E. Moerner, Ph.D., Harry S. Mosher Professor in Chemistry and Professor, by courtesy, of Applied Physics at Stanford University—worked out different approaches to achieve super-resolved fluorescence microscopy, which has enabled nanoscale imaging of living tissue (see Fig. 1) and innumerable benefits for life sciences and for society."This year's prize is about how the optical microscope became a nanoscope," said Staffan Normark, Permanent Secretary of the Royal Swedish Academy of Sciences, in announcing the academy's decision. Sven Lidin, Chair of the Nobel Committee for Chemistry 2014, said that with super-resolution microscopy, "guesswork has turned into hard facts, and obscurity has turned into clarity."
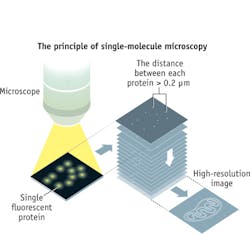
The prize recognizes two separate principles:
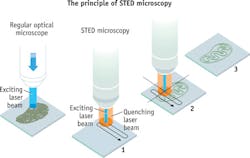
The first enables stimulated emission depletion (STED) microscopy, which Hell developed in 2000 (see Fig. 2).The Academy highlighted work that the researchers have pursued with the help of the imaging advance that they developed: the ability to follow individual proteins in fertilized eggs as they divide into embryos (Betzig); the understanding of how molecules create synapses between nerve cells in the brain (Hell); and the ability to track proteins involved in Huntington's disease as they aggregate (Moerner).
In fact, Moerner's most recent publication demonstrates use of super-resolution fluorescence to understand the mechanism underlying the progression of Huntington's disease and to designing therapeutics for the disease, as well as for aggregates implicated in Alzheimer's and Parkinson's diseases.1
And Hell, in delivering the plenary talk at LASER World of Photonics trade fair and World of Photonics Congress (May 13-16, 2013, Munich, Germany), said that nonlinear microscopy "is not the best line of thinking" to achieve nanoscale resolution. In fact, he presented the RESOLFT (REversible Saturable OpticaL Fluorescence Transitions) approach, which uses on-off switching of various states to enable imaging at molecular and even atomic scales.
A new imaging platform developed by Betzig and colleagues, announced on October 24, 2014, offers another leap forward (see "Laureate's reprise shows real-time subcellular activity in 3D"). Lattice light-sheet microscopy's ability to collect high-resolution images rapidly and minimize damage to cells means it can image the three-dimensional activity of molecules, cells, and embryos in fine detail over longer periods than was previously possible.
REFERENCE
1. W. C. Duim, Y. Jiang, K. Shen, J. Frydman, and W. E. Moerner, ACS Chem. Biol., doi:abs/10.1021/cb500335w (2014).

Barbara Gefvert | Editor-in-Chief, BioOptics World (2008-2020)
Barbara G. Gefvert has been a science and technology editor and writer since 1987, and served as editor in chief on multiple publications, including Sensors magazine for nearly a decade.