HIGH-SPEED IMAGING: Choosing components to image intracellular signals
All multicellular organisms more complex than sponges manage their complexity by transmitting signals through nerves. They act by propagating action potentials—waves of ionic concentration that carry information or trigger cellular events. In humans, action potentials govern the ability to detect the smell of fresh bread and the beating of our hearts. Many excitable cell types in the human body—including neurons, cardiomyocytes, muscle fibers, and pancreatic beta cells—need action potentials to properly function.1
Studying action potentials is integral to understanding the physiology of complex organisms. Understanding how each cell type responds to an action potential is critical for discerning the cell's physiological role and function, but action potentials are not easy to study. Cutting-edge research in areas such as synaptic activity and intracellular communication puts incredible demands on technology. For instance, action potentials typically oscillate at frequencies of 10–100 Hz, which means they must be imaged at least twice as frequently—at minimum 20–200 frames/s. Tracking the initial catalyst and propagation of an action potential requires even faster control: exposure and/or illumination times as short as 0.01–1 ms.
As we will discuss, new high-performance sensors, lenses, light modules, and other biophotonics tools are meeting these research needs.
A century of study
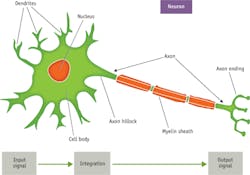
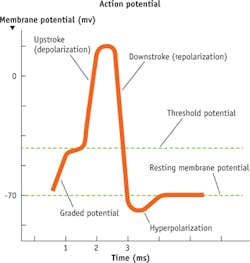
Scientists have been studying and documenting action potentials for well over 100 years. Their work has revealed that action potentials propagate and interact through special voltage-dependent channels along axons and in the cells with which they interact (see Fig. 1). These channels open and close to regulate the flow of ions, including sodium (Na+), potassium (K+), calcium, and chlorine (see Fig. 2). In axons, for example, when a trigger signal exceeds a specific threshold voltage, sodium channels open to allow the flow of positive sodium ions to the outside of a cell. The shift of charge increases the voltage difference until the channels close after a fixed interval. The result is a rapid and uniform electrical signal that conducts down to the axon terminal or along the membrane of a muscle fiber. These signals begin with sodium depolarization, have a positive feedback, and follow an "all-or-none" physiological affect.1Prior to 1955, experimental methods used to study action potentials were crude, unreliable, and suffered from two key problems. First, axons are extremely small and almost impossible to properly extract. Second, it was very difficult to isolate signals from single neurons or axons. Their internal voltages were recordable, but it was hard to analyze or acquire useful information from them.
The first problem was solved by extracting axons from giant squids. The second problem was solved with the development of voltage clamps and patch clamps-laboratory tools that allowed researchers to study ionic currents of action potentials in complete isolation, eliminating tremendous amounts of electrical noise. These solutions came hand-in-hand, as the inventions of the patch clamp and voltage clamp were dependent on the extraction of giant squid axons.
Analyzing action potentials with an analog or digital signal is relatively simple now, but actually visualizing them is still difficult. It has been proposed that action potentials are typically ~5 ms in duration at 10-100 Hz traveling at speeds up to 2.5 m/s.1 This presents a challenge: Above a frequency of 50 Hz, the quality of action potential images degrades because of poor signal-to-noise ratios (SNRs).
Choosing components to image the action
As when designing any imaging instrument, the first step in creating a system to image action potentials is clarifying requirements. The field-of-view requirement will drive the selection of objective lens and sensor format. Desired resolution and image acquisition time will be important for selecting your detector technology. And illumination flexibility is a major driver for selecting a light source.
To get started, define a general field of view so you can select an appropriate imaging lens or objective lens. Imaging the regeneration and recycling of vesicles in neurotransmitters requires a 2.5 × 2.0 μm field of view in the synapse with a frame rate of 28 frames/s. Although this small window is difficult to achieve with standard imaging components, the use of software with digital zoom and binning can get you close. The field of view with a 100X objective on a ½-in. sensor is roughly 60 × 50 μm; the field of view with a 200X objective on a ½-in. sensor is roughly 30 × 25 μm, and the field of view with a 400X magnification system on a ½-in. sensor is approximately 15 × 12.5 μm. Resolution is far beyond what you need to resolve these cells and their ongoing intracellular activity. One can easily resolve at the diffraction limit with these objectives, achieving 400 nm resolution or even greater depending on the light source and fluorescence emission signal.
Next, you'll want to examine your options for detectors. The two sensors that most researchers use when setting up a digital video microscope are charge-coupled device (CCD) and complementary metal oxide semiconductor (CMOS) detectors. The two types are similar, distinguished by minor material changes and details of their electrical configurations. Electron-multiplying CCD (EMCCD) and scientific CMOS (sCMOS) devices represent the power of these technologies, and are capable of producing quality images at high frame rates and low light levels.
In most imaging conditions, researchers find sCMOS detectors to be optimum. sCMOS detectors are multi-megapixel sensors that offer a large field of view with high resolution while maintaining good readout noise, dynamic range, and frame rate. They also offer rolling or global shutters to ensure the most versatile and powerful performance. Compared to an interline CCD or EMCCD, sCMOS detectors (such as the new Andor Zyla 4.2) can provide 4X more pixels, 5X greater sensitivity, 5X greater frame rates (all sustained at full frame), and 10X greater dynamic range. The major drawback of these powerful sensors is the price tag, as most easily exceed $15,000.2
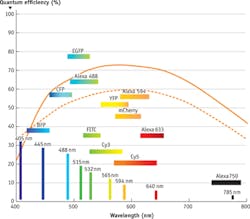
sCMOS sensors have high responsivity and quantum efficiency at critical UV and blue wavelengths for fluorophores, specifically around 388, 405, and 488 nm (see Fig. 3). You'll get the highest resolution, clearest images when you match the light source to the responsivity of the imaging sensor. Today, laser excitation sources are often chosen for these demanding microscopy applications, but there are still good reasons to incorporate a broadband white light source with high-transmission bandpass filters.2Lasers allow researchers to expose their sample to the maximum amount of energy at the specific wavelength required, but that same specificity keeps them from being as versatile as a broadband sources. Broadband white light sources can now achieve energy and power levels of lasers while remaining cool and safe for biological samples (avoiding photobleaching is critical). By incorporating fluorescent filter sets, you can tune a broadband white light source to a specific excitation line. The drawback of this configuration is that it's a bit more involved than a laser setup, and requires some level of optical or coating expertise. Ultimately, either a laser or filtered broadband white light source can maximize safe levels of excitation energy onto the sample, which the fluorophores convert to a strong, brief emission signal to be captured by the sCMOS sensor.
Bringing the options together The voltage-gated calcium (Ca2+) channel provides an important trigger for the action potential. Calcium channel signals and waves can be as fleeting as a few microseconds and as long as 2–3 hours. Here's an outline of how you might go about broadly defining the requirements for imaging this channel.
Pericams, cameleons, and camgaroos are fluorophores used to label calcium. These fluorescent calcium indicators are frequently used to visually track action potentials and intracellular activity. Other options, in the form of synthetic derivatives of these fluorescent protein reporters, include Fura-2, Indol-1, and fluo-4, which claim to be more durable and brighter. These fluorescent indicators are particularly useful for noninvasively measuring fleeting or small changes in Ca2+. The expression level is specific to a tissue region or transport system, but it is possible to monitor these on both the subcellular to multicellular level at high-speed or time-lapse intervals.3
The most important factors that determine fluorescence intensity include indicator concentration, excitation path length, and cytosolic localization. Each of these can vary, again, depending on the specific tissue region or transport system. Fluorescence intensity will also be affected by cell variability, or phenomena such as photobleaching, compartmentalization, or complete extrusion. Local conditions such as fluidity, viscosity, pH, and pressure can also affect Ca2+ indicator fluorescence. These and other factors make it critical to establish a baseline—the general level of fluorescence flux that naturally occurs due to cell to cell variability.
The ideal system for measurement of the Ca2+ indicators includes an excitation source of 340 or 380 nm (depending on the fluorescent protein bound to the calcium channel), which will result in a 405 or 475 nm emission signal. This excitation can come from a laser line and a dichroic/emission filter pair, or a broadband white light source with an excitation, dichroic, and emission filter trio. In either case, a sCMOS detector will be the recommended choice to measure the fluorescence signal, which will be brief and weak and thus require a high frame rate with extreme sensitivity. The optimum objective lens will depend upon the field of view necessary for the specific application.3
Thanks to technological advances, imaging of action potentials is possible today. Still, the endeavor remains a challenge. But if you clearly understand your requirements and select the components of your imaging system appropriately, your measurements can provide insight into this fertile field of research.
REFERENCES
1. D. U. Silverthorn, Human physiology, 4th ed., Pearson Benajim Cummings, New York, NY (2006).
2. See http://www.andor.com/pdfs/literature/Andor_sCMOS_Brochure.pdf.
3. See http://cshprotocols.cshlp.org/ content/2013/2/pdb.top066050.full.
Stephan Briggs | Biomedical Product Line Engineer, Edmund Optics
Stephan Briggs started with Edmund Optics as an intern while pursuing his degree in biomedical engineering at Drexel University with a concentration on tissue engineering and biomaterials. Following graduation, he joined as Biomedical Engineer, working directly with EO’s life sciences customers. With a solid understanding of processes and technologies, he helps researchers and system builders accomplish their goals with correct application and integration of components and subsystems. He has authored numerous articles on a range of topics of interest to life scientists, including light sources, optics, individual and combined imaging approaches, and cost-efficient design options.