OPTICAL COHERENCE TOMOGRAPHY: OCT for oncology: Preclinical progress highlights clinical potential
OCT-based imaging tools developed for preclinical intravital cancer imaging have yielded exciting new capabilities to probe and to monitor cancer progression and response in-vivo. These tools portend still more utility of OCT for preclinical work, and even for translation to clinical therapy monitoring.
EDITOR’S NOTE: This article is based on the paper by B. J. Vakoc, D. Fukumura, R. K. Jain, and B. E. Bouma, Nat. Rev. Cancer, 12, 363–368 (May 2012).
In addition to its application to an increasing number of diagnostic applications (including cardiology and endoscopy in addition to ophthalmology), optical coherence tomography (OCT) has made an important impact on preclinical work. In the past few years, exciting achievements in intravital cancer imaging—that is, imaging of tumors in living mouse models—have underscored a growing role for OCT. This noninvasive, label-free technology is providing new capabilities for imaging different aspects of the biology of tumors and their microenvironments, and is being integrated with complementary modalities to enhance information obtained from studies. These advances point to potential for using OCT to monitor the clinical treatment of cancer in the future.
Benchtop to endoscopy
An OCT system typically comprises a light source, an interferometer, and a microscope or imaging catheter that delivers light to, and collects reflections from, the tissue to be imaged. Because of optical scattering, OCT signals cannot penetrate beyond 2 mm in most tissues—a fact that necessitates careful selection of animal models. Often, as with fluorescence microscopies that have limited imaging depths, benchtop imaging is carried out using window models at the skin, mammary fat pad or brain sites.1-3
OCT can also be used for subcutaneous models by transdermal imaging and can be applied to surgically exposed internal sites.4 Benchtop use involves a microscope that is similar in design to those used in fluorescence microscopy, in which anesthetized animals are placed under the objective and galvanometric beam scanners translate the imaging beam.
Because OCT can be operated with low numerical aperture (NA) lenses, OCT optics can be integrated into small probes, catheters, and endoscopes or imaging at internal sites.5,6 Miniaturization does not typically induce substantial degradation in imaging sensitivity or resolution, and endoscopic embodiments are the most likely pathway for the potential future clinical adoption of OCT tools with which to monitor cancer therapy.
Image contrast applications of OCT
Fundamentally, OCT measures how light propagates in tissue and how it scatters from tissue structures. Over the past decade, our ability to translate these measurements into physiological and anatomical parameters has expanded considerably. Several OCT methods are relevant to oncology:
Microstructural imaging. Structural (anatomical) imaging is used to measure tumor volume, to locate tumors anatomically, or to define the tumor microenvironment. Intravital ultrasound and micro-computed tomography (μCT), both commonly used for this purpose, have the advantages of deep penetration and large imaging fields, but are hindered by fairly poor soft-tissue contrast.
Microstructural images can be generated from OCT measurements using the logscale magnitude of optical scattering. Because optical scattering is more varied across soft tissues than either acoustic scattering or X-ray absorption, microstructural OCT images generally provide greater contrast. This increases the ability to detect tumor margins, and more broadly elucidates the microenvironment at the tumor site—making OCT preferable for smaller tumors. Microstructural OCT imaging can be carried out rapidly: An 8 × 8 × 2 mm field at 10 μm resolution takes <5 s to acquire using current instrumentation.7Viability imaging. Imaging viability within a tumor model helps elucidate the spatially heterogeneous response to therapy, but methods for this purpose are limited. Micro-positron emission tomography (μPET), fluorescent deoxyglucose analogs, green fluorescent protein (GFP), and luciferase reporter bioluminescence have all been used, but each has a significant shortcoming. That is why this compelling application for OCT—which can discriminate between viable and non-viable compartments of a tumor through correlated changes in optical scattering.4
Although the underlying changes in tissue structure that modulate optical scattering have not been identified, a correlation between high scattering and loss of viability has been confirmed by registered images and histology. (Caution must be used when associating scattering changes to viability status, however, as other processes could manifest similar scattering changes.) OCT-based viability imaging has the advantage of being label-free, and can be easily carried out concurrently with other OCT imaging modalities—for example, with microstructural imaging, both contrast methods operate on the same acquired data set and are differentiated only by post-processing.
Lymphangiography. Lymphatic vessels have a central role in solid tumor growth and metastasis. The disruption of normal lymphatic function by solid tumors contributes to the high interstitial fluid pressure within the solid tumor and hinders convective drug transport. Conversely, peri-tumor lymphatic vessels provide a route for metastasis. There is, therefore, a need to image lymphatic vessels in solid tumor microenvironments and in response to cancer therapies. However, lymphatic imaging remains one of the most considerable challenges in intravital microscopy because, unlike the blood vasculature, the lymphatic system cannot be easily systemically labeled. The most common technique requires tracer injection into the site (for example, into the tumor or the surrounding tissue). As lymphatic vessels collect and are filled with the injected tracers, they can be imaged by either wide-field cameras or fluorescent microscopy. This approach, however, only reveals a partial network and it perturbs lymphatic vessel physiology.
OCT can be used for label-free lymphangiography.4,8 Contrast for the lymphatics is derived from the difference in optical scattering between lymph fluid, which is nearly transparent, and tissue, which is highly scattering. The lymphatic network appears in three-dimensional data sets as hypoechoic (low-scattering) regions; three-dimensional connectivity, as well as characteristic valve and lymphangion morphology, make the identification of lymphatics fairly straightforward. Because a proportion of the lymphatic vasculature is collapsed at any given time in normal physiology, comprehensive mapping is not always possible. Higher-resolution embodiments of OCT that are able to detect lymphatic vessels with smaller open luminal areas are technically feasible and would mitigate this limitation.
Angiography. Understanding tumor angiogenesis and the response of tumors to vascular-targeting therapies have been major themes in cancer research over the past decade. Current approaches for intravital angiography include Doppler ultrasound, micro-magnetic resonance imaging (μMRI), μCT, photoacoustic tomography, and fluorescence microscopy. Of these, ultrasound, μMRI, and μCT cannot resolve single vessels owing to limited resolution. Fluorescence has been broadly adopted to study tumor models at the resolution of individual vessels, but fluorescent methods require labeling through intravenous injection, which carries known limitations in longitudinal studies.2
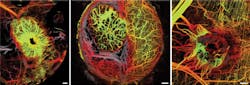
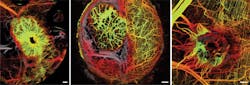
Microstructural OCT enables visualization of some large vessels (>100 μm diameter); to extend contrast to smaller vessels, alternative methods based on blood flow are commonly used in OCT. Unlike the contrast methods (microstructural, viability, and lymphatic) already discussed, angiographic imaging relies on the measurement of scattering dynamics. Flowing blood modulates optical scattering, and this modulation can be detected and used to differentiate between the intravascular and extravascular compartments of the tumor. Unlike fluorescence approaches, OCT-based angiography is label-free, while fluorescence microscopy provides smaller fields and higher resolution.
Also unlike the other contrast OCT methods, angiographic OCT monitors scattering changes over time and so requires strict animal immobilization and great care for doing so. It also requires longer imaging times to capture the scattering dynamics at each location (up to 20 minutes for an 8 × 8 × 2 mm field at 10 μm resolution, compared with ~5 s for non-dynamic contrast modes of OCT)-although algorithm refinements are likely to reduce this time requirement in the near future.4 However, longer imaging times allow for more extensive sampling of the signal dynamics, which leads to improved image contrast.
Additional contrast modes. Other modes include techniques for detecting exogenous labels, which could enable OCT-based measurements of drug distribution within tumors.9 Also possible is the use of OCT for identifying specific tissue components, such as collagen and muscle fibers, by measuring their influence on the polarization of the incident light field.10 This polarization-sensitive OCT approach is fairly well established, and may be useful for probing tumor-stroma interactions. Finally, careful measurements of scattering dynamics may yield contrast for cellular processes such as apoptosis or may allow for comprehensive quantification of blood flow velocity in a tumor vascular network.11
Demonstrated effectiveness
Investigators have adopted these OCT methods to study cancer biology and to evaluate drug response in preclinical settings. The usefulness of microstructural OCT has been demonstrated in several studies. For instance, it enabled researchers to delineate tumor margins in three dimensions and subsequently determine tumor volume, which facilitated definition of consistent therapeutic start points and measurement of response across animals in a drug study.4 In another study, microstructural OCT was used endoscopically to comprehensively map and longitudinally track disease progression in induced models of colorectal cancer.5 The ability of OCT to monitor cell-targeted therapy was demonstrated by viability imaging.4 And OCT-based lymphangiography has been used to map lymphatic vessels in the peri-tumor space in multiple models.4
Some of the most compelling applications of OCT in preclinical cancer research have been in the area of angiography. The ability of OCT to repeatedly image over broad fields makes it ideal for studies of tumor angiogenesis and vascular response across a variety of sites.4 And in an example of hybrid studies that combine OCT with other modalities, an instrument that was capable of dynamically imaging oxygen supply and demand in tumors was also demonstrated.12 In this model, OCT was used to measure blood flow while spectroscopic techniques provided measures of blood oxygenation (using hemoglobin spectroscopy) and metabolic demand (using 'redox ratio' fluorescence measurements).
The wide-field imaging of OCT reveals the morphological nature of tumor vascular networks with a unique clarity. This capability was used to highlight the important role of the microenvironment in tumor vasculature (see figure).4 Frequently repeated angiography—which is not practical using fluorescence microscopy owing to imaging time and accumulation of extravasated label—is possible using OCT.
Clinical translation
Despite their effectiveness, many preclinical imaging approaches are not translatable to the clinic because they rely on genetic modifications or because they would require regulatory approval of a new label. OCT has moved rapidly into diagnostic applications generally because it is label-free and thereby circumvents these translational challenges. With the growth in OCT-based imaging of cancer demonstrated in preclinical settings, there is a strong argument for efforts to translate OCT to clinical monitoring of cancer therapies. Early work in animal models has demonstrated that OCT-based angiography can provide sensitive feedback on the effect of photodynamic therapy in models of prostate cancer and esophageal carcinoma.13-16 Efforts to translate OCT techniques to clinical response monitoring are now underway.
REFERENCES
1. M. Leunig et al., Cancer Res., 52, 6553–6560 (1992).
2. E. Brown, L. L. Munn, D. Fukumura, and R. K. Jain, Cold Spring Harb. Protoc., 7, doi:10.1101/pdb.prot5452 (July 1, 2010).
3. S. Hak, N. Reitan, O. Haraldseth, and C. de Lange Davies, Angiogenesis, 13, 113–130 (2010).
4. B. J. Vakoc et al., Nat. Med., 15, 1219–1223 (2009).
5. A. Winkler et al., Mol. Imaging Biol., 13, 1173–1182 (2010).
6. A. Winkler, P. Rice, R. Drezek, and J. Barton, J. Biomed. Opt., 15, 041512 (2010).
7. C. T. Badea, M. Drangova, D. W. Holdsworth, and G. A. Johnson, Phys. Med. Biol., 53, R319-R350 (2008).|
8. Y. Jung, Z. Zhi, and R. Wang, J. Biomed. Opt., 15, 050501–050503 (2010).
9. S. A. Boppart, A. L. Oldenburg, C. Xu, and D. L. Marks, J. Biomed. Opt., 10, 041208 (2005).
10.J. de Boer and T. Milner, J. Biomed. Opt., 7, 359–371 (2002).
11. G. Farhat et al., J. Biomed. Opt., 16, 070505 (2012).
12. M. Skala et al., J. Biomed. Opt., 15, 011112 (2010).
13. B. A. Standish et al., Cancer Res., 68, 9987–9995 (2008).
14. B. A. Standish et al., J. Biomed. Opt., 12, 034022 (2007).
15. B. A. Standish et al., J. Gastrointest. Endosc., 326–333 (2007).
16. H. Li et al., Lasers Surg. Med., 38, 754–761 (2006).
More BioOptics World Current Issue Articles
More BioOptics World Archives Issue Articles