LIVE CELL IMAGING/DRUG DELIVERY: Nanowire + optical fiber = hi-res intracellular imaging probe
A nanowire-based endoscope system is enabling fluorescence study of biological processes within single living cells in high spatial and temporal resolution. It also promises delivery of genes, proteins, and therapeutic drugs without damaging cells, and could be applied to biosensing and single-cell electrophysiology.
Researchers at the U.S. Department of Energy (DOE)'s Lawrence Berkeley National Laboratory (Berkeley Lab; Berkeley, CA) and the University of California–Berkeley created the probe by attaching to the tapered end of an optical fiber a tin oxide nanowire waveguide able to manipulate light at the nanoscale. Light traveling along the optical fiber can be coupled into the nanowire, where it is re-emitted into free space when it reaches the tip.1
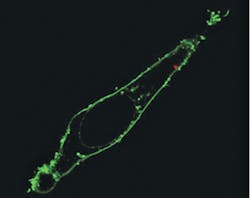
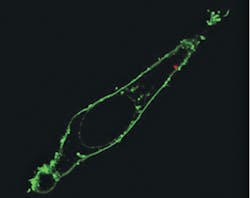
To test the probe as a local light source for subcellular imaging, the team optically coupled it to an excitation laser and then waveguided blue light across the membrane and inside individual HeLa cells. The cells were injured by neither insertion of the nanowire, nor by the light's picoliter-scale illumination volume. Also, because output from the endoscope was closely confined to the nanowire tip, it offered highly directional and localized illumination, says Peidong Yang of Berkeley Lab's Materials Sciences Division, who led the work. The team has shown that their endoscope can also detect optical signals from subcellular regions.
To deliver payloads into specific intracellular locations, the researchers attached quantum dots to the nanowire tip using photoactivated linkers. Confocal microscopy scanning of the cell confirmed delivery past the fluorescently labeled membrane and into the cytoplasm, says Yang, and photoactivation had no significant effect on cell viability. Yang says that his team's nanowire endoscope could also be used to electrically or optically stimulate a living cell someday.
The blue laser light was used to excite one of two quantum dot clusters located 2 μm apart. With the tight illumination area and small separation between the light source and the dots, low background fluorescence and high imaging contrast were ensured.
1. R. Yan et al., Nat. Nanotechnol., doi:10.1038/nnano. 2011.226 (2011).
More BioOptics World Current Issue Articles
More BioOptics World Archives Issue Articles