ONCOLOGY/FLUORESCENCE IMAGING: Seeking a one-size-fits-all probe for fluorescence-guided surgery
The ability to make cancerous tissue glow promises great advantages for tumor detection during fluoresence image-guided surgery. In one study, patients with malignant gliomas were given 5-aminolevulinic acid three hours prior to surgery. About half the patients (139) underwent fluorescence-guided neurosurgery, while the other half (131) had standard bright-light surgery. Of those receiving the fluorescence-enhanced intervention, 65% displayed complete removal of their tumors, while only 36% of the bright-light patients showed complete tumor resection. And the fluorescence approach continued to pay benefits down the road: The fluorescence patients had nearly doubled (41%) the 6-month progression-free survival as those who had surgery under white light (21%).1
But coming up with a broadly applicable means for labeling tumors has proven a bit tricky, as demonstrated by a report in Science Translational Medicine.2 An exciting new method developed by Urano and colleagues to label tumors by topically spraying with a tumor-specific probe has the advantage of being quick (seconds to minutes) and easy—and in experiments with living mice, allowed laparoscopic removal of implants as small as 1 mm under fluorescence guidance.3 But the application of this approach is unfortunately limited to tumors that overexpress gamma-glutamyl transpeptidase (GGT).
Likewise, the report notes, the targeting of folate receptor-α (FR-α) facilitated real-time multispectral intraoperative fluorescence imaging and resection of tumor deposits of less than 1 mm, but it too may be limited because overexpression of FR-α varies greatly among different tumors types. And the results of study mentioned above notwithstanding, 5-aminolevulinic acid may have limited labeling applicability beyond glioma.
Methods that use fluorescent tumor-specific antibodies may have wider applicability—but antibodies require intravenous injection, and labeling takes hours or days. Furthermore, this method is limited to cancers for which tumor-specific antigens have been characterized. Use of activatable cell-penetrating peptides (ACPPs) is effective, too—but only in tumors that express an appropriate protease with which to cleave and thus activate the probe.
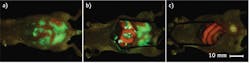
Because tumors of all types express telomerase, the genetic labeling method that uses a telomerase-dependent adenovirus to deliver GFP specifically to tumors offers the potential of widespread application. This genetically stable labeling system also allows detection of cancer recurrence: Any residual disease remains labeled and can be further resected, offering a potentially a huge advantage—especially when coupled with fluorescence laparoscopy.4
However, the telomerase-dependent adenovirus must be further tested for safety in humans before this method can be translated.
1. G. M. van Dam et al., Nat. Med., 17, 1315–1319 (2011).
2. M. Bouvet and R. M. Hoffman, Sci. Transl. Med., 3, 110fs10 (2011).
3. Y. Urano et al., Sci. Transl. Med., 3, 110ra119 (2011).
4. H. Kishimoto et al., Cell Cyc., 10, 2737–2741 (2011).
More BioOptics World Current Issue Articles
More BioOptics World Archives Issue Articles