BIOINSTRUMENTATION/BIOIMAGING: Whole-slide specimen scanning for digital analysis
The world of whole-slide imaging is growing increasingly digital. Rather than painstakingly peering through a microscope for days, weeks, or months to document features in slides, researchers from basic biologists to pathologists are using automated scanning to create digital files that can be analyzed and shared around the world. Among the approaches to biomedical slide scanning are three we'll explore—Leica's Total Digital Pathology (TDP), the VS120 from Olympus, Prior Scientific's slide loaders, and Hamamatsu's NanoZoomer—which are part of an expanding array of automated scanning tools.
While regulatory approval will be required for use of these tools in primary diagnosis, the approach has many other potential applications—some of which are not yet fully understood: Digitization is powerful because it enables quantification and detailed examination of many details in many slides, it accommodates results from virtually all forms of biological microscopy, and it facilitates collaboration.
Scan, manage, and analyze
In discussing TDP, Donal O'Shea, Ph.D., head of digital pathology at Leica Microsystems, headquartered in Heerbrugg, Switzerland, describes three key steps behind whole-slide imaging: Scan, manage, and analyze. The scanning creates a digital file from a microscope slide, the managing step helps a researcher organize the information, and analysis pulls information from the slides.
Scanning the slides at high resolution generates large files. "It's like looking at each blade of a grass on a football pitch," says O'Shea. "Each image makes a file that is 250 megabytes to 2 to 3 gigabytes in size." Consequently, just 1,000 images—maybe fewer—can fill a terabyte of storage, so this technology requires some knowledge of the information technology (IT) implications. As a result, notes O'Shea, "You need scalable storage solutions, usually in a server environment."
Various companies provide platforms that help researchers automatically scan many slides. For example, the PL-100 and -200 slide loaders from Prior Scientific (Rockland, MA) handle 100 or 200 slides, respectively. Moreover, these devices can be easily interfaced with a variety of microscope platforms and software packages to scan slides in batches.
Although whole-slide imaging depends on sophisticated technology under the hood, so to speak, vendors must make these tools accessible to a wide range of users. For example, O'Shea points out that users of Leica's TDP include educators teaching pathology, academic researchers, and pharmaceutical scientists—plus clinicians sharing images for consultations. Consequently, says O'Shea, "We focus hugely on usability, because the users are often non-technical from an IT perspective."
For research applications, says O'Shea, "This technology enables translational research, like looking at the localized expression of biomarkers in diseased tissue versus normal." Moreover, Leica's newest image-analysis software, Tissue IA 2.0, provides colocalization capabilities that can determine if two biomarkers interact in some way.
Manipulating the modalities
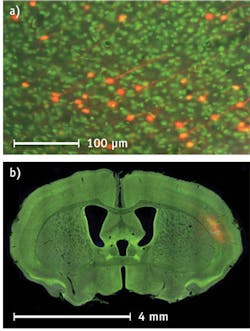
The microscope-based VS120 from Olympus America (Center Valley, PA), helps a researcher switch among slide-scanning modalities at magnifications from 2 to 100x. Christopher Higgins, the company's group marketing manager for clinical digital imaging, also points out the fluorescence option for the VS120. "In the brain, for instance, you could do a bilateral injection of a virus and map the activity of neurons on both sides. To see the structure of a given brain section and observe where this activity is in the brain, a counterstain of Nissl will highlight cell-body groupings, but the addition of darkfield scanning adds all the myelin structures, tissue outline, and more." Consequently, the scanner's software lets a researcher combine several methods of imaging.
Although the VS120 technology, as Higgins describes it, "makes the user intimate with the scope, you don't need to know every step." Instead, "wizards" in the software guide the user seamlessly through the process. As Higgins explains, "You might start by capturing a roadmap of the slide at 2x." That roadmap can be scanned in brightfield, darkfield, or fluorescence. "Later, you might use fluorescence to determine where your fluorophores are active, and go to that tissue," he says. "You have the option of viewing in any of the imaging modes, and you can also change the magnification." As a researcher makes these changes during scans, the software keeps track and aligns everything correctly. The system even automatically adjusts the exposure as needed.
According to Higgins, the software is robust and allows imaging of complex and difficult samples. "For instance," he says, "you can follow axons in and out of fiber tracts within the brain." Even with these vast capabilities, Higgins says that he can have a new user up and running in just a couple of hours. "With a little practice, almost anyone can quickly be scanning whole slides."
Zooming in and out
In describing the NanoZoomer, Scott Blakely, manager of sales and marketing of whole-slide imaging at Hamamatsu (Bridgewater, NJ), says, "The NanoZoomer is the perfect balance between ease of use and superior image quality for digitizing any or all areas on a slide." He adds: "Using a secure image server, the digitized slides can then be easily shared with others." Consequently, the NanoZoomer is being used for medical, dental, and veterinary education, plus research, archiving, and non-diagnostic consultations.
As an example of this technology's capabilities, Blakely points out that the NanoZoomer 2.0-HT can scan an image of an entire brain slice from a mouse and then zoom in on individual neurons. Moreover, a user can load up to 210 slides to be scanned automatically. The scanner also provides a user with a choice of scanning at 20x or 40x, which provide resolutions of 0.46 or 0.23 µm/pixel, respectively. The NanoZoomer can also acquire multiples scans of thick tissue in the z-axis.
This scanner also works in brightfield and an option adds a fluorescent mode. For brightfield, the NanoZoomer scans an area of 15 × 15 mm at 20x in about one minute. The same area takes about 2.5 minutes to scan at 40x. The scan times for fluorescence depend on the quality of the sample, the amount of dye present on the sample, the number of dyes used, and other factors.
Applications and potential
Although digital approaches to pathology require regulatory approval for some applications, such as primary diagnosis, this approach promises to keep expanding to new areas. For example, working from a digitized file, researchers can quantify information more accurately than ever before and also examine far more details in more slides. Consequently, this technology pulls more information from virtually any form of biological microscopy. Moreover, as developers explore new analytical techniques and include them in software updates, even more advanced analysis will become possible.
Likewise, the opportunities to collaborate should not be ignored. Sharing microscopy images around the world so quickly and easily unveils entirely new options of how scientists go about their work. Only time—and more digital scanning—can show us the extent of changes that this technology will spawn.
Mike May | Contributing Editor, BioOptics World
Mike May writes about instrumentation design and application for BioOptics World. He earned his Ph.D. in neurobiology and behavior from Cornell University and is a member of Sigma Xi: The Scientific Research Society. He has written two books and scores of articles in the field of biomedicine.