OPTICS/SOFTWARE/FLUORESCENCE MICROSCOPY: Tools for 3-D imaging
The shape of most optical lenses provides symmetry. If you slice a lens through the middle—top to bottom—one side looks like the mirror image of the other. That consistent shape, though, does not intrigue Allen Yi, associate professor of integrated systems engineering at Ohio State University in Columbus. Instead, he seeks asymmetry. He says, “I’m interested in something that would be unique or different.” That interest lured him to freeform optics and applying them to 3-D imaging.
After working in industry at Corning Precision Optics, a Cincinnati-based company purchased by 3M Company (NY: MMM) in 2002, Yi built an academic lab that explores asymmetrical optical lenses. With this approach he hoped to make a 3-D lens in one piece, rather than using the multiple lenses typically employed. In the December 2010 Journal of the Optical Society of America, Yi and his postdoctoral researcher, Lei Li, described a freeform prism that turns an ordinary microscope into one that, in one shot, produces a 3-D image.
Yi and Li used computational ray tracing—essentially simulating how light passes through a material—to find the lens shape that they desired. Then, they turned to a commercial milling machine that uses a diamond blade to cut the lens from a transparent plastic, called polymethyl methacrylate. This machine shaves the plastic down, 10 nm at a time. “We’re still having trouble creating fundamentally different processes to make these lenses,” says Yi, “so we’re still applying older processes.” The resulting lens looks a bit like a cut stone for jewelry, with a faceted top and a wide, flat base. It works like many lenses at once, each facet grabbing light from a different view of a sample (see Fig. 1).
“With freeform,” says Yi, “you get a very compact design.” In fact, Yi and Li’s lens can be attached just below the objective of an ordinary microscope. This lens is considered asymmetrical because, as Yi says, “you cannot find an axis that you can rotate the lens around and find symmetry.”
Yi and Li designed this lens to focus on objects about 1 mm in size, which covers plenty of interesting biology. For example, Yi aimed this lens at teeth from bats. “It’s difficult to use a regular camera for these teeth and impossible to use optical coherence tomography (OCT),” he says. With the freeform lens, Yi notes: “It forms pretty decent images.” He hopes to make even better ones by processing the images with software.
Going inside
Beyond looking at a single sample like a bat’s tooth, biologists simultaneously explore multiple items. For example, Martin Daffertshofer, global product manager, imaging software for PerkinElmer (NYSE: PKI; Waltham, MA), says, “In a three-dimensional matrix of cells, you need 3-D microscopy to identify and understand intracellular and intercellular relationships.”
In essence, matrices of cells make up biology. Imagine looking for an infection in such a matrix. “The virus might be rare,” says Daffertshofer. “Take malaria, for instance, which comes from just one viral entry.”
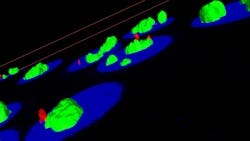
To look for malaria in a collection of cells, a researcher can add a fluorescent label that attaches to the virus, take an image with, say, a confocal microscope, and then use PerkinElmer’s Volocity 6.0 software (see page 37) to interpret a 3-D image. Daffertshofer describes this software as “a universal solution for 3-D image analysis.” To automate this process, a biologist can adjust the software to pick out cells with defined characteristics, such as a particular level of fluorescence in the nucleus or a particular morphology. If the researcher applies two fluorescent dyes, Volocity can even see if they occur together, which simplifies studies of the colocalization of proteins (see Fig. 2).As Daffertshofer points out, the 64-bit capability of Volocity even makes it possible to track cells in large images. Furthermore, a researcher can use this software with high-content screening systems like PerkinElmer’s Opera or Operetta.
Turning this technology into results, however, depends on ease of use. The latest version of Volocity aims at simplification. “What took five or six steps before now just takes two or three to get a very complete picture,” says Daffertshofer.
Focusing with phase
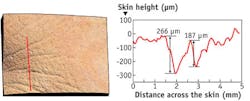
Sometimes, today’s 3-D imaging reminds us of the past. In 1887, for instance, German-American physicist Albert Michelson and American chemist Edward Morley looked for interference fringes—the fuzzy bands caused from the interaction of in- and out-of-phase light—to measure the speed of light. Today, Christian Benderoth, general manager, life sciences at GFMesstechnik (Teltow, Germany), and his colleagues create 3-D images with phase-shifted fringe patterns. In short, these scientists project striped patterns of light—fringes—onto a surface. The 3-D structure of the surface distorts the fringes, which get recorded with a camera (see Fig. 3).This technology collects height measurements for points on the surface—all in less than a second. To make a precise fringe pattern, GFMesstechnik uses digital micro mirror display technology, called DLP, from Texas Instruments (Dallas, TX). In some cases, the GFMesstechnik device can provide height resolution of <1 µm.
In most instances, GFMesstechnik applies this technology to skin. “You can quantify skin roughness, as well as complete body shapes from different fields of view,” says Benderoth. “This can be used in cosmetic and reconstructive surgery or to measure skin roughness before and after wound treatment.”
The GFMesstechnik device can also lay the 3-D information over a color photo (see Fig. 4). As Benderoth says, “It’s often important to see color changes in the skin.”As scientists combine old and new approaches, small changes could generate even better 3-D views of biology. “For optics,” says Yi, “only a tiny difference lies between a good and bad image.”
Mike May | Contributing Editor, BioOptics World
Mike May writes about instrumentation design and application for BioOptics World. He earned his Ph.D. in neurobiology and behavior from Cornell University and is a member of Sigma Xi: The Scientific Research Society. He has written two books and scores of articles in the field of biomedicine.