OPTICAL COHERENCE TOMOGRAPHY/TISSUE PHANTOMS: A physical eye model for OCT
T. SCOTT ROWE
In the field of ophthalmology, standard, well-characterized "test eyes" (tissue phantoms) are generally available to enable development and comparison of diagnostic instruments. The corneal topographer, the refractometer, and, more recently, the wavefront aberrometer all have the benefit of readily available test eyes designed to help evaluate their application performance. The need for a standard test eye is now becoming apparent as well for ophthalmic optical coherence tomography (OCT) instruments. Such a model would have many uses, including:
a) A target for demonstrating OCT functionality and imagery. A standard model or "eye" allows for instruments to be compared and features demonstrated, for instance, at trade shows.
b) A target for training OCT operators, technicians, and repair personnel, freeing up others from having to serve as a target while the technician learns. Studies show that well-trained ophthalmic technicians produce consistent measurements.
c) A target for OCT researchers and engineers, allowing for the development and calibration of automatic layer segmentation software with known retinal layer dimensions. Eye safety issues are not a concern with an eye model/tissue phantom.
d) A target for evaluating clinical systems from multiple manufacturers for clinical trial purposes. It has been well documented4,7,8,10 that clinical systems from different manufacturers—particularly time domain vs. spectral domain—do not match in terms of RT measurements. A "standard" model eye that is easily shipped could be used to correlate instruments of differing technologies and manufacturers.
Clearly defined criteria
For the development of tissue phantoms in general—and eye model tissue phantoms in particular—it is helpful to be clear regarding purpose. The more detailed and precise the definition for use, the better the chance of success: General eye models and phantoms rarely meet any serious commercial or research requirements.
In developing a novel test eye11 for ophthalmic OCT, we determined that a tissue phantom of a normal human retina would need to be sufficiently detailed to allow for unambiguous imaging, and replicate the architecture and morphology of the fovea, with sufficient layer definition to allow for automatic layer segmentation by the instruments—thus allowing for retinal thickness (RT) measurements, an important clinical measurement. Initially, the field of view could be limited to 18° (<5 mm in diameter), but the phantom would have to be embedded in an eye model that would enable the rapid alignment and acquisition of the phantom. The eye model should also be constructed such that it would not require fluids that need to be cleaned or maintained: ideally, it would be a solid-state construction.
Tissue optics and phantom design
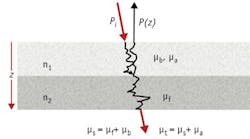
Building a tissue phantom for OCT with proper optical properties necessitates consideration of how the OCT signal is generated. The following equation describes in one dimension the intensity of backscattered light as it propagates through tissue:5, 6
where Γ is a function depending on source coherence, Pi is the incident beam intensity at the tissue, R is the Fresnel reflection from a tissue interface, μb is the backscatter cross-section coefficient, μt is the total attenuation scattering coefficient, and μt = μa + μs, δ(z-zn) is a delta function (depicting location of reflecting and scattering layers).
The two key elements for phantoms are the backscatter coefficient and the interface reflection. They work together to define the power of backscattered light that the OCT collects and, in order to achieve a realistic intensity, the signal return from the phantom depends on this combination of backscatter and reflection being equivalent to the modeled tissue. A key point to make here on the Fresnel reflection is that at any tissue interface involving different indices of refraction n1 and n2, R = (|n1 - n2|/(n1 + n2))2 at normal incidence. Thus, it is possible to construct phantom interfaces using optical materials with absolute indices different to those being mimicked, as long as their adjusted Rs are close (see Fig. 1). Accommodation will need to be made for the differences in optical path lengths (thicknesses) in the design, but this opens up a much wider range of materials than what is normally discussed for tissue phantoms.
A major goal in designing this retinal phantom is to achieve good backscattering properties for each layer from an OCT perspective. We assigned the following morphology:
a) A total retinal thickness (RT) of ~300 μm. This would put it in the range of normal eyes measured from the inner limiting membrane (ILM) to the top of the retinal pigment epithelium (RPE).7,8
b) A foveal pit of ~120 μm deep, and a maximum diameter of 1.2 mm3. This would leave a central retinal thickness of ~180 μm deep, again within the range of normals.9,10
c) An RT comprised of five layers of 60 μm each. Although not exactly corresponding with all of the neurosensory retinal layers, it comes close, and each layer in this stack can be adjusted for either reflectivity (R) or backscatter (μb).
d) An RPE with relatively high R and μb, typically 60–80 μm thick. Later designs attempt to separate the inner segments/outer segments (IS/OS) from the RPE with a separate layer.
e) A choroid with very similar R and μb to a human choroid, able to change absorption to explore use at wavelengths longer than 1060 nm.Current revision and future directions
After much experimentation and eye model imaging,1,2 we have achieved improved results in the morphology and the combination of backscatter and reflections of these phantoms. We are now able to achieve differentiation between the closely aligned IS/OS layer and the RPE layer, and to "tone down" the bright reflection, to at least within an order of magnitude of actual retinal layer tissue reflectivity (see Figs. 4, 5, and 6).ACKNOWLEDGEMENTS
The author would like to thank Dr. Robert Zawadzki of the UC Davis Department of Ophthalmology and Vision Science for his many helpful suggestions and test images during the development of this eye model and tissue phantom.
References
1. R. J. Zawadzki, T. S. Rowe, A. R. Fuller, B. Hamann, and J. S. Werner, "Toward building an anatomically correct solid eye model with volumetric representation of retinal morphology," Proc. SPIE, 7550, 75502F (2010).
2. R. J. Zawadzki, T. S. Rowe, and J. S. Werner, "Progress Report on Building an Anatomically Correct Solid Eye Model with Volumetric Representation of Retinal Morphology," 2010 ISIE Conference, Fort Lauderdale, FL (May 1, 2010).
3. A. M. Dubis, J. T. McAllister, and J. Carroll, Brit. J. Ophthalmol., 93, 1223–1227 (2009).
4. G. Vizzeri et al., Brit. J. Ophthalmol., 93, 775–781 (2009).
5. J. M. Schmitt, IEEE J. Select. Topics Quantum Electron., 5, 4, 1205–1215 (1999).
6. J. M. Schmitt, A. Knüttel, and R. Bonner, Appl. Opt., 32, 6032–6042 (1993).
7. S. Grover, R. K. Murthy, V. S. Brar, and K. V. Chalam, Am. J. Ophthalmol., 148, 266–271 (2009).
8. S. Grover, R. K. Murthy, V. S. Brar, and K. V. Chalam, Invest. Ophthalmol. Vis. Sci., 51, 2644–2647 (May 2010).
9. P. H. Tomlins and R. K. Wang, J. Phys. D: Appl. Phys., 38, 2519–2535 (2005).
10. I. Krebs et al., Invest. Ophthalmol. Vis. Sci., 52, 6925–6933 (August 2011).
11. T. S. Rowe, "Phantom For Rendering Biological Tissue Regions", U.S. Pat. 8,480,230 (2013).
T. Scott Rowe is principal/chief consultant with Rowe Technical Design, Inc.; e-mail: [email protected]; www.rowetechnical.com.

![FIGURE 2. A normal human retina,11 imaged using a high-resolution OCT (~4 μm axial resolution) system, is represented in this B-scan slice with each layer identified (a). The B-scan was derived from 3D data sets (b, c). Abbreviations: FoH - fibers of Henle; GCL - ganglion cell layer; INL - inner nuclear layer; IPL - inner plexiform layer; ISL - inner segment layer; NFL - nerve fiber layer; ONL - outer nuclear layer; OPL - outer plexiform layer; OSL - outer segment layer; RPE - retinal pigment epithelium. The outer limiting membrane (OLM; sometimes called external limiting membrane [ELM]), connecting cilia (CC; sometimes called inner/outer segment junction), Verhoeff's membrane (VM; sometimes called cone photoreceptor outer segment tips [COST]), and rod photoreceptor outer segment tips (ROST) may also be seen. FIGURE 2. A normal human retina,11 imaged using a high-resolution OCT (~4 μm axial resolution) system, is represented in this B-scan slice with each layer identified (a). The B-scan was derived from 3D data sets (b, c). Abbreviations: FoH - fibers of Henle; GCL - ganglion cell layer; INL - inner nuclear layer; IPL - inner plexiform layer; ISL - inner segment layer; NFL - nerve fiber layer; ONL - outer nuclear layer; OPL - outer plexiform layer; OSL - outer segment layer; RPE - retinal pigment epithelium. The outer limiting membrane (OLM; sometimes called external limiting membrane [ELM]), connecting cilia (CC; sometimes called inner/outer segment junction), Verhoeff's membrane (VM; sometimes called cone photoreceptor outer segment tips [COST]), and rod photoreceptor outer segment tips (ROST) may also be seen.](https://img.laserfocusworld.com/files/base/ebm/lfw/image/2015/12/1311bowrowef2.png?auto=format,compress&fit=max&q=45&w=250&width=250)
![FIGURE 3. In the retinal tissue phantom design (shown in [a] en face and [b] cross-sectional views), five layers of 60 μm uniform thickness are stacked to create a 300-μm-thick RT. The foveal pit also mimics normal foveal pit morphology. FIGURE 3. In the retinal tissue phantom design (shown in [a] en face and [b] cross-sectional views), five layers of 60 μm uniform thickness are stacked to create a 300-μm-thick RT. The foveal pit also mimics normal foveal pit morphology.](https://img.laserfocusworld.com/files/base/ebm/lfw/image/2015/12/1311bowrowef3b.png?auto=format,compress&fit=max&q=45&w=250&width=250)


